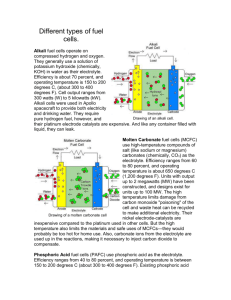
j
advertisement

j Architectures for Individual and Stacked Micro Single Chamber Solid Oxide Fuel Cells by Ethan Jon Crumlin B.S., Mechanical Engineering Massachusetts Institute of Technology, 2005 Submitted to the Department of Mechanical Engineering in Partial Fulfillment of the Requirements for the Degree of Masters of Science in Mechanical Engineering at the Massachusetts Institute of Technology June 2007 ©2007 Massachusetts Institute of Technology All rights reserved Signature of Author 4e Certified by....... 7V/ Accepted by.................... ............ MASSACHUSETTS INSTITUTE TCH 0 etOf Mechanical Engineering May 19, 2007 . . . . . . . . . . ....... . ........ Yang Shao-Horn Assistant Professor of Mechanical Engineering Thesis Supervisor .. ......~~~~~~....... ........................... Lallit Anand Professor of Mechanical Engineering Chairman, Department of Graduate Committee MASSACHUSETTS INST!TUTE OF T~C;H A. L~o B~ARKER JUL 13 207 Architectures for Individual and Stacked Micro Single Chamber Solid Oxide Fuel Cells by Ethan Jon Crumlin Submitted to the Department of Mechanical Engineering On May 19, 2007 in Partial Fulfillment of the Requirements for the Degree of Masters of Science in Mechanical Engineering ABSTRACT Solid oxide fuel cells (SOFCs) are electrochemical conversion devices that convert various fuel sources directly into electrical energy at temperatures ranging from 600'C to 1000'C. These high temperatures could potentially allow the direct use of various hydrocarbon fuel sources and hydrogen, without the need for expensive noble metal catalysis. Conventional SOFCs are designed in a two-chamber system, separating the fuel and oxidant flow to the anode and cathode, respectively. However, fuel cell manufacturing cost and robustness have proven to be the main challenges to rapid commercialization. A promising alternate method to achieve these requirements and to open up new architecture designs for the SOFC is the development of single-chamber solid oxide fuel cells (SC-SOFCs). SC-SOFCs avoid many of the manufacturing challenges associated with conventional SOFCs, and have shown optimal performance between 500*C and 800'C. This reduces the need for high temperature sealing and a complicated manifold structure; however it also reduces the partial pressure of the gases at the electrodes, which reduces the theoretical obtainable voltage. Microfabrication techniques such as photolithography, sputtering, and photo-resist liftoff were used to create various micro SC-SOFC that are 25-400microns long and 15-40microns wide, utilizing platinum and gold for the electrodes and YSZ as the electrolyte. After successfully fabricating these micro SC-SOFCs, the fuel cells were tested in a microprobestation with a custom gas chamber enclosure, which was exposed to CH 4 :0 2:N 2 at 20:20:100 ccm or 40:20:100 ccm. A switch in the OCV from a negative voltage to a positive voltage was observed around 600'C, possible indicating change in electrochemical reactions with temperature. An OCV of -0.4V and peak power density of 27 [W/cm 2 at 900'C in a 1:1 methane:oxygen ratio was achieved. A stack of 10 micro SC-SOFCs as fabricated showing a cumulative OCV of 3.3 V, of an average 0.33 V per cell at 600'C in a 2:1 methane:oxygen ratio. Ongoing research will involve characterizing micro SC-SOFCs to understand the fundamental reaction mechanisms, electrode materials, and architectures to obtain dense, high performing stacks of micro SC-SOFCs. Thesis Supervisor: Title: Yang Shao-Horn Assistant Professor of Mechanical Engineering 4 LIST OF PUBLICATIONS 1. Crumlin, E. J., G. J. la 0', and Y. Shao-Horn, Architectures and Performance of High-Voltage, Microscale Single-Chamber Solid Oxide Fuel Cell Stacks, the Electrochemical Transactions, in press (2007). 2. la 0', G.J., H.J. In, E. Crumlin, G. Barbastathis and Y. Shao-Horn, Recent Advances in Microdevices for Electrochemical Conversion and Storage, invited review paper, International Journal of Energy Research, 31, 548-575 (2007). 5 6 ACKNOWLEDGMENTS I would like to give my sincere gratitude to my family. Mom, dad and bud: you have always been there to support me through the hard times and share with me the moments of success. You have loved me, shaped me, guided me to become the person I have become. Thank you and I love you all deeply. Professor Yang Shao-Horn: you are my research advisor, academic advisor, and personal advisor. I thank you immensely for your guidance, wisdom, challenges, and most important, your respect. You have helped me control and grow my strengths as well as pushed me to improve my weaknesses. Your effort, patience, candidness, intellect, wisdom, and energy are all characteristics I admire and benefit from. I look forward to working with you in many more endeavors. To my fellow lab mate, graduate student Gerardo Jose la 0': thank you for all of your guidance, advice. Thank you for always being there to help even when it was inconvenient for you. You are a great person to learn from and work with, and I hope we can do so for a long time to come. A special thanks to Kurt Broderick, one of the key support staff at MIT's Micro Technology Laboratory; thank you for all your help with the fabrication involved in this thesis. Thanks to all of my lab members for their continuous support and effort to help me move forward with my research. In addition, I would like to thank all of my college mentors who have provided continuous guidance and support throughout all of my undergraduate and graduate career. Professor Kip Hodges, Professor Alex Slocum, Professor David Wallace, you have all helped me reach the goals and gain the experiences I have hoped to achieve. Thank you very much for everything. I would like to extend a special thanks to Lu Chen for editing my thesis and putting a smile on my face everyday. This work was supported by the GEM fellowship, MIT-Lemelson Fund, Ford-MIT Alliance, MRSEC Program of the National Science Foundation under award number DMR 02-13282. 7 8 ABOUT THE AUTHOR I was born in Northampton, Massachusetts, on May 6, 1983. I attended Flag Street Elementary School in Worcester, Massachusetts where I developed my interest in science and engineering by participating in my school's science fair and winning 3 out of 4 years. After the 6 th grade, I attended Forest Grove Junior High School, where for three days a week, Principle Joseph Murphy would drive several of us to the local high school for a special math class. Junior high is when I started to do Tae Kwon Do and developed an interest in music. I spent two years at Doherty Memorial High School where I played football for two years. I also joined a swim team, became a lifeguard, and taught swim lessons. After two years I attended a focused junior-senior high school, Massachusetts Academy of Mathematics and Science. My science and engineering abilities where nurtured and developed through academics as well as extracurricular activities, namely, FIRST. Through participating in FIRST, I learned how to work in a machine shop, use engineering principles and techniques to design and build. This experience was a fundamental tool that helped me truly excel and learn on my own. In 2001, I graduated from high school and moved to Cambridge, Massachusetts to study Mechanical Engineering at MIT. I have felt both humbled and honored during my time at MIT; learning discipline and control, while absorbing the tools and resources to excel in my field. During the summers, I have held internships with Intel in Phoenix, Arizona, Texas Instruments in North Attleboro, Massachusetts, and Apple, Inc (for three summers) in Cupertino, California. Upon graduating in 2005 with my Bachelor of Science degree in Mechanical Engineering, I decided to pursue further academic goals at MIT. During the last two years while working towards my Masters of Science degree in Mechanical Engineering, my academic focus has been on new alternative energy technologies, such as various low temperature and high temperature fuel cell projects, one of which is the culmination of my master's thesis research to follow. Professionally I focus on product design; I founded a startup company, OneWorld Medical Devices, which I led to become Runners-Up in MIT's $100k business-plan competition. I was also recruited to join another startup company, RallyPoint, where I am the lead mechanical engineer for an intelligent device for your hands. I have carried my passion for product design towards teaching others as well. I have mentored or been a lab instructor for a senior product design class (2.009), sophomore design class (2.007), and a toy design class. Looking ahead, I am planning to obtain my PhD with the hopes of becoming a professor at a leading institution such as MIT. Additionally, I'd like to continue my entrepreneurial efforts and create new startups to continue working on a variety of new products and innovations to enhance our lives. 9 10 LIST OF FIGURES Figure 1: A traditional two chamber fuel cell assembly comprised of two electrodes on either side of an electrolyte. For SOFCs the cathode distributes electrons to reduce oxygen to make an oxygen anion. The anion travels through the electrolyte to the anode where it oxidizes with the fuel and donates the electron, completing the 24 electrochem ical reaction. ..................................................................................... Figure 2: High performing SOFC with 5micron thick LSGM film electrolyte using a 400nm thick SDC buffer layer to interface electrolyte and electrode. Using H2 and 02 over anode and cathode respectively at a flow rate of 120mL/min. (7)............. 25 Figure 3: Single chamber solid oxide fuel cell (SC-SOFC)........................................... 25 Figure 4: Two chamber SOFC results (a) SC-SOFC results (b) (41). ............................ 26 Figure 5: An example of proposed electrochemical reactions for a SC-SOFC exposed to 27 methane and oxygen............................................................................................. Figure 6: Planar SC-SOFC Architecture....................................................................... 29 Figure 7: Through-Plane SC-SOFC Architecture........................................................ 29 The difference between active area of Planar and Through-Plane Figure 8: configurations. The right most image compares the relative size of each active area and shows that the area of the Planar configuration is more than two times that of 30 the Through-Plane configuration. .......................................................................... Figure 9: The effect of changing the distance between electrodes in a planar SC-SOFC configuration as shown in Figure 6. Note that the wider the gap the worse the 32 perform ance (19)................................................................................................. Figure 10: Analytical model predicting the effect of distance between two electrodes (a) and electrolyte thickness (b) on planar SC-SOFC performance (13). .................... 33 Figure 11: Planar SC-SOFC varying electrolyte thickness. If the electrolyte is too thin the fuel cells performance could be reduced. If it is too large, fuel cell performance is not significantly affected. (figure is representative, not actual experimental or . . 34 analytical values)............................................................................................... Figure 12: The effect of electrolyte thickness on performance in a through-plane SCSOFC configuration shown in Figure 7. Note increasing thickness decreases . . 35 perform ance (19)................................................................................................. Figure 13: Schematic for stacking SC-SOFCs .............................................................. 35 Figure 14: Schematic for individual micro SC-SOFC test cells..................................... 38 Figure 15: Schematic for planar single (a) or multiple (b) interconnect micro SC-SOFC 39 stac k ......................................................................................................................... Figure 16: Steps for micro SC-SOFC fabrication........................................................ 40 Figure 17: Typical IV graph for SOFC operating at 8000C(5)...................................... 41 13 Figure 18: Simplified fuel cell circuit diagram where R,, and C. represent the effects imposed by the electrolyte and R2 and C2 represent the electrode effects (a). Ideal ac impedance plot for the circuit above (b) (46).................................................... 43 Figure 19: An individual micro SC-SOFC with 15micron by 400micron Pt and Au electrodes in 1:1:5 methane:oxygen:nitrogen fuel ratio, OCV at various temperatures (a), current-voltage at various temperatures (b), current density-power density at various temperatures (c),....................................................................... 46 Figure 20: Au and Pt electrodes on YSZ electrolyte upon a silicon substrate at 400'C exposed to various gas compositions: Nitrogen (a), Oxygen (b), Methane (c), Methane:Oxygen:Nitrogen in a 1:1:5 volume flow rate ratio(d)............................ 48 Figure 21: Au and Pt electrodes on YSZ electrolyte upon a silicon substrate at 850'C exposed to various gas compositions: Nitrogen (a), Oxygen (b), Methane (c), Methane:Oxygen:Nitrogen in a 1:1:5 volume flow rate ratio(d)............................ 49 Figure 22: Individual micro SC-SOFC with 5micron wide Pt and Au electrodes separated by a gap distance of 15micron and exposed to methane:oxygen:nitrogen in a 1:1:5 fuel ratio at 900C, average OCV as a function of fuel cell area holding the electrode width constant at 5microns and varying the electrodes length (a) current-voltage (b) current-power (c)................................................................... 52 Figure 23: Individual micro SC-SOFC with 5micron wide Pt and Au electrodes separated by a gap distance of 5micron and exposed to methane:oxygen:nitrogen in a 1:1:5 fuel ratio at 900*C, average OCV as a function of fuel cell area holding the electrode width constant at 5microns and varying the electrodes length (a) currentvoltage (b) current-pow er (c)................................................................................ 53 Figure 24: Two separate trials for various 'PtAu' width electrodes that are 400micron long exposed to 1:1:5 ratio of methane:oxygen:nitrogen at 700 0 C, average electrode resistances (a), average electrolyte resistances (b). ................................................ 55 Figure 25: Various 'PtAu' width electrodes that are 400micron in length exposed to 2:1:5 ratio of methane:oxygen:nitrogen at 600'C and 700'C, average electrode resistances (a), average electrolyte resistances (b). ................................................ 56 Figure 26: Various 'Pt _Au' width electrodes that are 100micron, 200micron and 400micron in length exposed to 2:1:5 ratio of methane:oxygen:nitrogen at 600 0 C, average electrode resistances (a), average electrolyte resistances (b). .................... 57 Figure 27: 5micron wide Pt and Au electrodes separated by a gap distance of 5micron, 10micron, and 15micron exposed to 2:1:5 ratio of methane:oxygen:nitrogen at 600 0 C, average electrode resistances (a), average electrolyte resistances (b)......... 58 Figure 28: 10 cells connected in series measuring the overall OCV as well as the OCV between the first 8, 6, 4, 2 and 1 cells. Each individual cell is 400micron long with 5micron wide electrodes, exposed to 2:1:5 methane:oxygen:nitrogen at 600'C......... 59 Figure 29: CHEMKIN thermodynamic equilibrium simulation over various temperatures for a 1:1:5 ratio of methane:oxygen:nitrogen..................................... 61 14 TABLE OF CONTENTS A b stract........................................................................................................................... List of Publications ................................................................................................... Acknowledgm ents...................................................................................................... About the Author ..................................................................................................... L ist o f F ig ures ............................................................................................................... L ist o f T ab le s ................................................................................................................ 3 5 7 9 13 19 1.0 21 21 22 22 22 In tro d u ctio n .......................................................................................................... 1.1 Motivation ............................................................................................. 1.2 Solid Oxide Fuel Cell Fundamentals ........................................................ 1.2.1 Solid Oxide Fuel Cell ................................................................. 1.2.2 Thermodynamics of Electrochemical Reaction .......................... 1.2.3 Traditional Two-Chamber SOFC Architecture and Performance ... 23 1.3 Single-Chamber Solid Oxide Fuel Cell ................................................... 25 1.3.1 Single-Chamber SOFC Concept ............................................... 25 1.3.2 Design of Electrode Activity and Selectivity for SC-SOFCs.....27 1.4 Single-Chamber Solid Oxide Fuel Cell Architectures................................ 29 1.4.1 Planar Design ............................................................................ 29 1.4.2 Through-Plane Design............................................................... 29 1.4.3 Comparing Planar vs. Through-Plane ........................................ 30 1.5 Relating Electrode and Cell Configurations to SC-SOFC performance.........31 1.5.1 Electrolyte Resistance................................................................. 31 1.6 Single-Chamber Solid Oxide Fuel Cell Stacking ....................................... 35 2.0 Experimental........................................................................................................... 37 2.1 Material Selection and Material Synthesis for micro-SC-SOFCs..............37 2.2 SC-SOFC Design..................................................................................... 37 2.2.1 Individual SC-SOFC design ..................................................... 37 2.2.2 Stacked M icro SC-SOFC Design............................................... 38 2.3 SC-SOFC Fabrication.............................................................................. 39 2.4 Experimental Setup................................................................................... 41 2.4.1 Testing Conditions and Apparatus ............................................. 41 2.4.2 Current-Voltage Data Collection ............................................... 41 2.4.3 AC Impedance Spectroscopy ...................................................... 42 2.4.4 Scanning Electron Microscopy (SEM) and Transmission Electron M icroscopy (TEM ) ........................................................................... 44 2.4.5 X-ray Diffraction........................................................................ 44 3 .0 Resu lts .................................................................................................................... 45 3.1 Individual Micro SC-SOFC Operation at Various Temperatures .............. 45 3.2 Micro SC-SOFC Electrode Changes at Various Temperatures and Gas Compositions.................................................................................................. 47 3.3 Single M icro SC-SOFC Performance........................................................ 51 3.4 AC Impedance Spectroscopy: Further Micro SC-SOFC Characterization and Isolation of Electrode and Electrolyte Effects ................................................. 54 59 3.5 Stacked SC-SOFC Data ........................................................................... 11 4.0 Discussion...............................................................................................................61 4.1 OCV verse Temperature: Electrode Catalytic Activity Reversal................61 66 4.2 Electrode Transform ation........................................................................ 76 4.3 Carbon N anowire Form ation.................................................................... 4.4 Characterization of Individual m icro SC-SOFCs....................................... 84 84 4.4.1 Current-Voltage Data ................................................................. 86 4.4.2 AC Impedance Data ................................................................... 91 4.5 Stacked SC-SOFC scaling........................................................................ 93 5.0 Sum mary................................................................................................................. 97 References .................................................................................................................... 103 Appendix .................................................................................................................... A. Temperature Dependence on Ion Conductivity for Selective Oxides ........... 103 12 Figure 30: Reaction mechanisms for micro SC-SOFC w/ gold and platinum electrodes in a 1:1:5 methane:oxygen:nitrogen mixture at >700*C include water-gas shift reaction of CO, H2 is oxidized on the platinum electrode, while 02 is reduced on th e A u electrode. ...................................................................................................... 63 Figure 31: Platinum, palladium and iridium CO to CO 2 formation rates as a function of inverse temperature for single crystal supported catalysts (50)..............................64 Figure 32: Reaction mechanisms for micro SC-SOFC w/ Au and Pt electrodes in a 1:1:5 methane:oxygen:nitrogen mixture at <600'C include water-gas shift reaction for the methane, carbon monoxide blocks the platinum electrode which promotes oxygen reduction, while carbon monoxide is oxidized on the gold electrode.........65 Figure 33: SEM images documenting the transformation of Pt-YSZ-Au micro SCSOFC after testing at 900C in a 1:1:5 ratio methane:oxygen:nitrogen gas mixture. Individual micro SC-SOFC(a), 5 400micron long micro SC-SOFCin series with single interconnect (b), different sized single and multi-interconnect series stack micro SC-SOFC(c), multi-interconnect series stack of micro SC-SOFC(d)...........67 Figure 34: SEM and EDS results to investigate platinum electrode. SEM image of porous platinum electrode indicating where EDS measurement is measured on the platinum surface(a), EDS results for measurement over platinum surface confirming the presence of platinum(b), SEM image of porous platinum electrode indicating where EDS measurement is taken within a platinum pore(c), EDS results for measurement within platinum pore, showing no evidence for platinum w ithin the pore(d)................................................................................................. 68 Figure 35: SEM and EDS results to investigate platinum diffusion into YSZ. SEM image of YSZ where platinum electrode had been; box indicates area within which EDS is measured (a), EDS results for measurement YSZ surface, showing no evidence for platinum (b) ..................................................................................... 69 Figure 36: Micro SC-SOFC exposed to methane at 850*C for over one hour................ 71 Figure 37: XRD results for samples with gold and platinum on YSZ and exposed to pure methane, pure oxygen, pure nitrogen and a 1:1:5 ratio of methan:oxygen:nitrogen. XRD results only detect platinum, gold and YSZ......71 Figure 38: Optical images of Pt-Au patterned directly onto silicon with no electrolyte, exposed to pure nitrogen at various temperatures. Individual micro SC-SOFCs at 400*C no visible changes observed (a), individual micro SC-SOFCs at 700*C initiation of platinum transformation (b), series stacks of micro SC-SOFCs at 700C more consistent changes occur to the platinum(c), individual micro SCSOFCs at 800'C more platinum and all of the gold starts to transform(d), series stacks of micro SC-SOFCs at 800C all platinum and gold transform (e-f). .......... 73 Figure 39: Optical images of Pt-Au patterned directly onto silicon with no electrolyte, exposed to a 1:1:5 ratio of methane:oxygen:nitrogen at various temperatures. Individual micro SC-SOFCs at 400C no visible changes observed (a), individual micro SC-SOFCs at 700 C platinum appears to deform (b), series stacks of micro SC-SOFCs at 700'C all platinum in contact with gold does not deform(c), series 15 stacks of micro SC-SOFCs at 750'C platinum in contact and within a certain distance of gold does not transform(d), individual micro SC-SOFCs at 800'C more platinum and all of the gold starts to transform(e), series stacks of micro SC-SOFCs at 800'C all platinum and gold transform (f-g)...................................................... 74 Figure 40: XRD results for samples with gold and platinum deposited directly on silicon (no YSZ electrolyte) and exposed to pure nitrogen or a 1:1:5 ratio of methan:oxygen:nitrogen. XRD indicates the existence of polycrystalline Pt, Au, 75 and the formation of a new substance PtSi............................................................ Figure 41: SEM images of the carbon nanowire formation on the surface of the sample with platinum being the catalysts. At a magnification of 250, it is apparent that platinum electrodes on all individual micro SC-SOFCs have been utilized to form the carbon nanowires (a) at higher magnification of 1000, there is no platinum remaining on the electrolyte, and gold is unaffected (b) at a magnification of 4000, it becomes clear that there is a tangled web of carbon nanowires(c)...................... 77 Figure 42: TEM image of a small platinum particle with a single carbon nanowire growing from its surface. The solid layers of carbon prove the formation of a 79 carbon nanowire verses a carbon nanotube. ........................................................... Figure 43: TEM image of a large platinum nano particle with multiple carbon 80 nanow ires grow ing from its surface. ...................................................................... Figure 44: SEM and EDS results to investigate platinum and carbon nanowires. SEM images of gold electrode, YSZ electrolyte and the transformed platinum electrode with carbon nanowires (a), EDS results taken from the platinum and carbon nanowire, showing carbon and platinum (gold is shown because the energy levels for gold and platinum are very similar and the software has trouble distinguishing between the two) (b), depicts the atomic ratio of elements present, indicating 81 substantially more carbon than platinum(c).......................................................... Figure 45: CHEMKIN thermodynamic equilibrium simulation over various 82 temperatures for a 2:1:5 ratio of methane:oxygen:nitrogen..................................... Figure 46: Power density versus current density for 5micron wide electrodes separated by a gap distance of 15microns, experimented at 900*C in a 1:1:5 84 m ethane:oxygen:nitrogen environm ent. ................................................................ Figure 47: Power density versus current density for 5micron wide electrodes separated by a gap distance of 5microns, experimented at 900*C in a 1:1:5 84 m ethane:oxygen:nitrogen environment ................................................................. Figure 48: Power density vs. fuel cell area. For each electrode gap distance, there is a given power density that is within the same range for the different fuel cell sizes. . . 85 Figure 49: A side by side comparison of Figure 9 and Figure 12 showing IV data for 86 planar (a) and through-plane(b) SC-SOFCs(19).................................................... Figure 50: resistance ratios with respect to electrode gap separation............................ 16 87 Figure 51: Analytical model of a micro SC-SOFC with 5micron and 15micron wide gold and platinum electrodes. The parameters for this model are based on extracted ASR values for the platinum and gold electrodes at 700*C. .................................. 89 Figure 52: Experimental data for a micro SC-SOFC with 15micron wide gold and platinum electrodes at 700'C in a 1:1:5 ratio of methane:oxygen:nitrogen. ............ 89 Figure 53: OCV across different quantities of micro SC-SOFCs stacked in series........ 91 Figure 54: average OCV across different quantities of micro SC-SOFCs stacked in serie s........................................................................................................................9 2 17 18 LIST OF TABLES Table 1: Overall Chemical Reaction and Change in Gibbs Free Energy for Methane, Hydrogen and Carbon Monoxide in a stochiametric mixture with oxygen at 30*C. 23 Table 2: Anode and Cathode electrochemical half cell reactions and overall electrochemical reaction for Methane, Hydrogen and Carbon Monoxide in a stoichiom etric mixture with oxygen................................................................... 27 Table 3: Individual micro SC-SOFC, with YSZ thickness of 200nanometers and conductance of 0.0035Q-'cm' at 600 0 C (43), at varying electrode lengths and separation distances ........................................................................................... 32 Table 4: AC Impedance experimental nomenclature................................................. 43 Table 5: micro SC-SOFC electrode transformation summary..................................... 50 Table 6: Thermodynamic equilibrium of gas species in the chamber with initial starting ratio of 1:1:5 methane:oxygen:nitrogen for 500'C and 800 0 C............................62 Table 7: Mole fraction of the primary gas species at thermal equilibrium for a 2:1:5 mixture of methane and oxygen in nitrogen at 800'C.........................................78 Table 8: Computed values for the change in resistance and the ASR.........................88 Table 9: Comparison of electrode and electrolyte resistances.................................... 90 19 20 1.0 INTRODUCTION 1.1 Motivation As our world becomes increasingly integrated with ever more powerful mobile electronics, providing enough energy to run these devices become increasingly difficult. The theoretical capacity limit for a lithium ion battery is about 400Wh/kg (1), and the current state-of-the-art batteries have the ability to reach the 150Wh/kg (1) threshold. Electronic devices will eventually require energy densities unreachable by lithium ion battery systems. Development of small fuel cells with liquid fuels could potentially provide 3-5 times more energy than batteries (2). This dramatic improvement in energy storage density is the primary motivation for fuel cell research for portable electronics products. The motivation for solid oxide fuel cell (SOFC) research is in its potential for high power densities and efficiencies and the intent for stationary applications or integration into a power generation cycle. SOFCs are high temperature (800*C-1 100*C) solid state energy conversion devices. The electrolyte is composed of an oxide ceramic material and is used to transport oxygen anions from the cathode to the anode, thus completing an electrochemical reaction and providing electrical energy in the process. Despite being able to provide high power densities at high efficiencies, SOFCs have several significant disadvantages. At such high operating temperatures, the types of materials that can be used for structure and sealing are very limited and costly. In addition, the sealing process at such temperatures is very complex and at least 2 seals per cell are required in stacked SOFC systems. These material and process barriers prevent traditional SOFCs from being a commercially viable energy conversion technology. Traditional SOFCs are further discussed in Section 1.2. The aforementioned shortcomings of traditional SOFCs provide great motivation for the research and development of single-chamber solid oxide fuel cells (SC-SOFC) which will be discussed in detail in Section 1.3. The objective for SC-SOFCs is to leverage the 21 benefits of traditional SOFCs with a simple innovative design concept, which possesses a dense stacking density, simplified architecture, ability to conform to countered surfaces, and lower operating temperatures (400-700*C (3, 4)). With these advantages, SC-SOFC technology can be implemented to run portable electronics for a longer period of time or be embedded into the exhaust stream of conventional power generation systems to recover and utilize un-reacted fuel. 1.2 Solid Oxide Fuel Cell Fundamentals 1.2.1 Solid Oxide Fuel Cell A SOFC is an electrochemical conversion device which operates at high temperatures of 800-1100 0 C (5). The high operating temperatures allow the usage of many different fuels such as hydrogen and various hydrocarbons, which react with oxygen to complete the electrochemical reaction. Even carbon monoxide can serve as a fuel at high temperatures, whereas at low temperatures, it would poison a PEMFC. SOFCs do not require noble metal catalysts when operating at these elevated temperatures. SOFCs are primarily composed of an oxide material. At the cathode, a reduction reaction occurs that transforms oxygen into an oxygen anion. This oxygen anion then transports through the solid oxide electrolyte to the anode, where it oxidizes the fuel, which gives off an electron and completes the electrochemical reaction (5). 1.2.2 Thermodynamics of Electrochemical Reaction Obtaining energy from any system is accomplished from a change in energy states. Table 1 provides the overall chemical reactions that occur within the fuel cell as well as the change in Gibbs Free Energy from this reaction and the corresponding equilibrium voltage. 22 Table 1: Overall Chemical Reaction and Change in Gibbs Free Energy for Methane, Hydrogen and Carbon Monoxide in a stochiametric mixture with oxygen at 30'C. Fuels Methane Hydrogen Carbon Monoxide Overall CH4 +202 -2H20+C02 H2+ Reaction 1O2 -> H20 C+102 -1 C02 AG -818 kJ/mol -237 kJ/mol 2 -257 kJ/mol E 1.41 V 1.23 V 1.33 V 2 The electrical potential can be evaluated with the Nernst equation, Eq. 1 (6): Erxn = E +-In RT zF E0 H [Hrea avJ AG zF Eq. 1 Eq.2 where E0 is the standard equilibrium voltage, Eq. 2 (6), at standard pressure of latm and is based off of the change in Gibbs Free Energy of the reaction shown in Table 1, R is the gas constant, T is the reaction temperature in Kelvin, z is the number of electrons being transferred, F is Faraday's constant, and within the brackets are the products of the reactants and products activities. For an ideal gas, the activity is equivalent to the partial pressure; therefore, the lower the partial pressure, the lower the open circuit voltage (OCV). The OCV for an SOFC operating at around 800'C is about 0.98 V, and decreases at higher temperatures and increases at lower temperatures. The decrease in OCV at elevated temperatures is compensated for by an increase in kinetics, making noble metals unnecessary for sufficient performance (5). 1.2.3 Traditional Two-Chamber SOFC Architecture and Performance Traditional SOFCs have two chambers, one that isolates and exposes the fuel to the anode, and another that isolates and exposes oxygen to the cathode, as shown in Figure 1. Traditional SOFCs have several benefits and drawbacks from their design. Some of the benefits as previously mentioned are increased rate or reactions, no need for noble metals, and high obtainable power densities of -2-3 W/cm 2 (7). However, these 23 results are difficult to achieve as the high operating temperatures demand very sturdy structure and seals. All of the materials need to match in thermal expansion to reduce thermally induced stresses and strains, which create cracks and limit the fuel cell's life. Additionally, if an SOFC electrode area were scaled up to 100cm 2 or larger, the solid oxide layers would be very thin and fragile and would be extremely susceptible to defects, cracking. Thus, it would be very difficult to create a mobile system, and SOFC technology would be limited to stationary applications (5). 02 Fuel 02 H201 Co 2 Figure 1: A traditional two chamber fuel cell assembly comprised of two electrodes on either side of an electrolyte. For SOFCs the cathode distributes electrons to reduce oxygen to make an oxygen anion. The anion travels through the electrolyte to the anode where it oxidizes with the fuel and donates the electron, completing the electrochemical reaction. For these reasons, there is a large push to reduce the operating temperature for SOFCs to 800*C or lower. An innovative sandwiching technique used for the electrolyte layer improves interfacial bonding and increases power density. This technique yields over 3.25W/cm 2 at an operating temperature of 700*C (Figure 2), thus providing relatively high performance at lower temperatures (7). 24 1 .0~ 3500 0000 * 0 0.8 ~0-0 290D 0.8 0 * .e 0 . o0 0600rC 00 00 1*9* OA 0.2 I A: 00 50 1W I 0. 0.0 0 100( 20W0 300 4000 MDW XMt dnweny (nWarn Figure 2: High performing SOFC with 5micron thick LSGM film electrolyte using a 400nm thick SDC buffer layer to interface electrolyte and electrode. Using H2 and 02 over anode and cathode respectively at a flow rate of 120mL/min. (7) 1.3 Single-Chamber Solid Oxide Fuel Cell 1.3.1 Single-Chamber SOFC Concept One way to further simplify SOFC architecture and thus avoid sealing issues and complex gas manifolds is to create an SC-SOFC. Figure 3 shows the SC-SOFC places the entire fuel cell into one chamber and simultaneously feeds fuel and oxygen. The idea behind the SC-SOFC is that the cathode and anode will have different catalytic selectivities, where the cathode is designed to primarily reduce oxygen and the anode designed to primarily oxidize the fuel (3, 4, 8-42). Fuel+0 2 CO2 + H20 Figure 3: Single chamber solid oxide fuel cell (SC-SOFC). 25 Placing the same fuel cell into a two chamber experimental setup and into a singlechamber experimental setup will help frame the effects from the different conditions. Using methane as the fuel source, Figure 4 shows the I-V curves for the operation of the fuel cells at various temperatures (41). In both fuel cell configurations, OCV decreases as temperature increases, as predicted by the Nernst equation, Eq. 1. Additionally, in the SC-SOFC, the oxygen and fuel gases are mixed; as the reaction proceeds, the product gases for both oxidation and reduction reactions act to decrease partial pressure of each species. Thus the extent of reaction in a SC-SOFC is less than that in a traditional SOFC, and therfore, the OCV of a SC-SOFC is lower than that of a two-chamber SOFC. In addition, the two-chamber SOFC does not show a plateau in performance with respect to temperature while the SC-SOFC seems to reach its maximum performance at around 500 0C. 800 1200 350 1000 1000 80300 250 600 600 200 400 600 5 40010 0 0 400 800 1200 Current density / mA cm 1600 2000 2 a 0 0 200 MC00 600 400 0 000 Current density / mA cmi b Figure 4: Two chamber SOFC results (a) SC-SOFC results (b) (41). This shows that for a SC-SOFC, there may be an optimal operating temperature that is lower than 800'C. Although the decreased operating temperature may eliminate sealing complications and allow better integration into mobile devices, the draw is in the decreased actual power density. This SC-SOFC has been shown to produce a max power of just over 300mW/cm 2 , while the two-chamber SOFC easily surpasses 1 W/cm2 (41). However, comparing the two fuel cell set ups at the low operating temperature of 500'C, the SC-SOFC has about 20% higher power output than the two-chamber SOFC (41). This suggests that if SOFCs are to operate at low temperatures, the more efficient way to do so would be by using a SC-SOFC. 26 1.3.2 Design of Electrode Activity and Selectivity for SC-SOFCs. With SC-SOFCs, the fuel and oxidant are simultaneously introduced into the chamber. This results in a decrease in activities of the reacting chemical species, which according to the Nernst equation, Eq. 1, would decrease fuel cell performance. However, this effect can be influenced by the catalytic selectivity of the electrodes. In an ideal SC-SOFC, the electrodes have complete selectivity, where each electrode participates only in its designated reaction. Figure 5 shows that the SC-SOFC cathode selectively reduces oxygen and does not react with the methane, while the anode facilitates the oxidation of the fuel, as well as potential gas shift reactions, but does not react with oxygen (23). Table 2 details the ideal half reactions at each electrode. jMi6 PF 2CH4 4#20 402 0, CH t >~ .,'~ Cathode 2 CH +402= 4H2 +2C,6--, . .....Anode -Electrolyte Figure 5: An example of proposed electrochemical reactions for a SC-SOFC exposed to methane and oxygen. Table 2: Anode and Cathode electrochemical half cell reactions and overall electrochemical reaction for Methane, Hydrogen and Carbon Monoxide in a stoichiometric mixture with oxygen. Fuels Anode Half Cell Reaction Cathode Half Cell Reaction Overall Reaction -> 2H 20+ CH4 +402 CO2 +8e-_ 202+ 8e- -->402CH4 +20 2 Carbon Monoxide Hydrogen Methane -> 2H 2 0+ CO 2 2H 2 + 202- -+ 2H 20+ 4e- 2CO + 202- - 2CO 2 + 4e02+ 4e - 2H2 +0 2 - 202 02+ 2H20 4e 2CO+0 - 2 2022CO2 I 27 Incomplete electrode selectivity negatively impacts the OCV. The two main speculations for poor electrode selectivity are related to a geometrical effect and a material effect. Geometry is believed to influence the OCV when the electrodes are oriented such that the products of each electrode dilutes and reduces the reaction potential of the other electrode(8, 28, 42). Ahns, et.al. showed that an OCV ~0.35 V in a SC-SOFC can be obtained using interdigitated electrodes that have a gap spacing of 50microns (8). This effect is further supported by Jacques-Bedard, et.al., who shows that decreasing the separation distances between cathode and anode from 300microns to 40microns reduces the OCV from 0.98 V to an unstable OCV ranging from 0.4-0.7V (28). Both studies use the same anode (nickel-oxide), cathode (LSM) and electrolyte (YSZ). This work supports the idea that the SC-SOFC's geometry can influence the OCV and that mixing electrode products negatively influences the reactivity of the electrodes. Choice of electrode material can impact OCV in one of two ways: the electrodes may be extremely reactive with both the fuel and the oxidant, or not very reactive with either (17, 23). Hibino, et.al. shows that when using the same electrolyte, Pt/YSZ/Au obtains an OCV of less than 0.6 V while Ni/YSZ/LSM obtains an OCV of 0.8 V. This reduction in OCV can be caused by the material's insufficient ability to react with the gas species; Hibino et.al. showed that the conversion of methane using platinum was only ~4%, while that using nickel was more than 50% (17). The OCV is also affected if the electrodes are over reactive. A difference in reactivity at each electrode is what generates a potential, to achieve a high OCV, the cathode needs to have a higher rate of oxygen reduction than the anode, and anode needs to have a higher rate of fuel oxidation than the cathode; therefore, rapid reactions at both electrodes are non-conducive to generating a high potential. 28 1.4 Single-Chamber Solid Oxide Fuel Cell Architectures 1.4.1 Planar Design In planar SC-SOFC architecture, Figure 6, the anode and cathode are aligned on the same face of the electrolyte (20, 21). The advantage of a planar SC-SOFC is that it is the simplest architecture to manufacture since the electrodes can be visually aligned to each other. Figure 6: Planar SC-SOFC Architecture. 1.4.2 Through-Plane Design In a through-plane SC-SOFC architecture, Figure 7, higher performances can be achieved than in a planar configuration by minimizing the electrolyte thickness, which reduces the electrolyte resistance (19). However, this is more challenging to fabricate due to the difficulties in aligning the anode and cathode when they are not necessarily visible. To obtain a thin electrolyte, this design would require support by a thick anode or cathode. Figure 7: Through-Plane SC-SOFC Architecture. 29 1.4.3 Comparing Planar vs. Through-Plane Assuming the same cell size, materials, and process steps, the through-plane architecture provides a larger power density than a planar configuration. There are two contributing factors for this, the more trivial factor is how power density is calculated. Power density is based off of a two-dimensional area, which is the active area of the fuel cell. As shown in Figure 8, the planar configuration has its electrodes side by side, and its active area is effectively double the electrode area plus the spacing area between the two electrodes. In contrast, in the through-plane SC-SOFC configuration, the electrodes are on top of each other, consuming less two-dimensional space. Planar Configuration Active Area Comparison Through-Plane Configuration I I~ I I I I I I I I I' I I I I I I ] I I I I I 1 I I I I I ___ I Planar Area> 2x(Opposite Area) Top View Top View I E Cross Section View Cross Section View Anode Electrolyte Cathode Active Area The difference between active area of Planar and Through-Plane Figure 8: configurations. The right most image compares the relative size of each active area and shows that the area of the Planar configuration is more than two times that of the Through-Plane configuration. 30 1.5 Relating Electrode and Cell Configurations to SC-SOFC performance Changing the geometric characteristics for the SC-SOFC, such as the distance between electrodes and the thickness of electrolyte, results in different effects discussed in the following sections. The two main parameters of the planar configuration, Figure 6, are the distance between the electrodes and the electrolyte thickness. 1.5.1 Electrolyte Resistance Eq. 3 can be used to calculate the electrolyte resistance, R: AR =R Eq. 3 The symbols 1 and A are both geometric properties, where / is the distance oxygen anions have to travel, and A is the cross-sectional area available for anion transport. The conductance, o, for various high temperature electrolytes such as YSZ changes as a function of temperature, a plot of which is provided in Appendix A (43). For example, an individual micro SC-SOFC that is 400microns long, with YSZ thickness of 200nanometers and conductance of 0.0035Q-'cm-1 at 600'C (43), and whose electrodes are separated by 5microns, yields a resistance of 1.79x10 5 Q. Table 3 provides the resistance values for different electrode and separation distances. Two main trends are apparent: the larger the electrode area, the lower the resistance, and the smaller the gap, the smaller the resistance. Therefore in a planar SC-SOFC configuration, it is expected that the best performing fuel cell would consist of a long electrolyte layer with the electrodes placed as closely as possible without touching. 31 .......... Table 3: Individual micro SC-SOFC, with YSZ thickness of 200nanometers and conductance of 0.0035Q- cm' at 600 0 C (43), at varying electrode lengths and separation distances Electrode-Electrode Separation Distance 9 at 5micron Q at 10micron 9 at 15micron 400microns 1.79 e5 Q 3.58 e5 Q 5.37 e5 Q 100microns 7.15 e5 Q 1.42 e6 Q 2.15 e6 Q 25 microns 2.86 e6 Q 5.72 e6 Q 8.58 e6 Q Length 1.5.2 Previous Findings As shown in Figure 9, increase in gap distance between the electrolytes decreases performance (19). This result is in accordance with Eq. 3, since increased gap distance, 1, results in increased resistance, R, and thus decreased voltage. Figure 9 clearly shows that the changes in current and power are linearly affected by the change in the distance between electrodes. This effect is also demonstrated with an analytical model shown in Figure 10a (13). I (W 140 -0.5nim pp 80 400 60 40 200 -Wider 20 0 k) 0 200 410 600 - 3nim 00 Current density / mA cmi~ Figure 9: The effect of changing the distance between electrodes in a planar SC-SOFC configuration as shown in Figure 6. Note that the wider the gap the worse the performance (19). 32 An analytical model was created to determine if there was an optimum electrolyte thickness (13). A micro planar SC-SOFC system with electrodes of width of 20micron and a gap distance of 20micron has an optimum electrolyte thickness of about 80microns, as shown in Figure 10b (13). For this analytical model, it is assumed that Ni-GDC is used as the anode, and SSC-SDC is used for the cathode, each with 100nm diameter pore size. Additionally, hydrogen and oxygen are the fuel and oxidant, and the system operates under the temperature 500'C. However, this model should only be considered as a foundation for the idea that a thicker electrolyte for planar SC-SOFCs can provide improved performance, because as will be discussed later, micro SC-SOFCs have difficulty experimentally obtaining a conventional OCV as shown in this model. Micro SC-SOFCs have experimentally demonstrated an OCV closer to 350-400mV (44), while many larger SC-SOFCs have an OCV of 0.95V or lower (3, 4, 9, 16-23, 26, 45). Experimental results to be discussed later show the OCV to be much lower than that predicted by the Nernst Equation as shown in this model. *100 U 9S &b ( l ) '~tJ0 40)X KX) 1 S3A) 4000 1 V0 zoo .1$'0 0 W0 tl10 b a Figure 10: Analytical model predicting the effect of distance between two electrodes (a) and electrolyte thickness (b) on planar SC-SOFC performance (13). Electrolyte thickness can play a role in planar SC-SOFC performance because as thickness decreases, the oxygen anion flow can be hindered, preventing its ability to flow freely and quickly from the cathode to the anode. This idea is visually expressed in Figure 11, where the electrodes have the ability to handle a fixed exchange current. When the electrolyte, the carrier for the exchange current, is too thin, it reduces the amount of current that can travel from one electrode to the other. Increasing the 33 =_ _ - - _.. --- - -1 - - MM thickness of the electrolyte will increase the current flow; however, further increase in thickness will have diminishing improvements to fuel cell performance. The trend explained above and conveyed in Figure 10b can also be extracted from Eq. 3. In this case, the electrode length and electrolyte conductivity are fixed, but the cross-sectional area is changed. With these constraints, the electrolyte resistance is now inversely proportional to area: increasing area decreases resistance and vice versa. Additionally, when the cross-sectional area of the electrolyte gets too large, its ability to improve the fuel cell performance is negligible. Alternatively, when the cross-sectional area becomes too small, it quickly increases the resistance towards the limit of infinity. Too thin of an electrolyte Anode Optimal electrolyte thickness Cathode Electrolyte Very thick electrolyte Exchange Current Pathway Figure 11: Planar SC-SOFC varying electrolyte thickness. If the electrolyte is too thin the fuel cells performance could be reduced. If it is too large, fuel cell performance is not significantly affected. (figure is representative, not actual experimental or analytical values) In Through-Plane SC-SOFC configuration shown in Figure 7, the gap between the electrodes is controlled by the thickness of the electrolyte; therefore electrolyte thickness is the major geometric factor that contributes to the fuel cell performance. In this case, the thinner the electrolyte, the higher the performance (19). Figure 12 provides experimental data demonstrating the relationship between electrolyte thickness and fuel cell performance of through-plane configured SC-SOFCs. In this configuration, the resistance is directly proportional to the electrolyte thickness, which is the length anions have to travel, Eq. 3. This trend is reflected in Figure 12. Despite slight deviations, which can be associated with experimental error, the resistance is strictly linear with respect to the thickness (13, 19). 34 > 250-0_ 000 wo 'nitii thickhicker -0.6mmn T0 600 40 0 mmn Lhicktiess! 200 400 600 SWf I( )200 1400 Cunetii density / mA m - Figure 12: The effect of electrolyte thickness on performance in a through-plane SCNote increasing thickness decreases SOFC configuration shown in Figure 7. performance (19). 1.6 Single-Chamber Solid Oxide Fuel Cell Stacking Thermodynamically, a single fuel cell can obtain a maximum voltage of ~0.98 volts at 800'C regardless of its size (see Section 1.2.2 for OCV thermodynamic dependencies). Note that Eq. 1 contains no area or volume term to scale the voltage. Changing the physical size of the fuel cell contributes to the amount of current that can flow through the system, but to increase the system's voltage, individual cells need to be connected in series, which will contribute linearly to an increase in voltage (21, 26, 37). Figure 13 is a schematic depicting the SC-SOFC stacking connecting the anode of one cell to the cathode of another, resulting in additive voltages. Figure 13: Schematic for stacking SC-SOFCs 35 36 2.0 Experimental 2.1 Material Selection and Material Synthesis for micro-SC-SOFCs The primary materials utilized for the micro SC-SOFC are platinum and gold to serve as the anode and cathode respectively, and YSZ as the electrolyte. YSZ is selected for its well-known electrochemical properties, and while platinum and gold are not conventional high temperature fuel cell catalyst materials, they do possess different catalytic reaction properties which are desirable for high electrode selectivity. Platinum has a higher affinity for oxidizing the fuel, while the gold has the ability to reduce oxygen. In addition, since the electrodes are pure noble metals, they simplify the potential reaction mechanisms, and are physically easier to handle and process. To further improve micro SC-SOFCs performance, future studies will investigate more complex materials such as solarium doped ceria (SDC) as the electrolyte, nickel combined with SDC as the anode, and barium strontium cobalt iron (BSFC) as the cathode (4). 2.2 SC-SOFC Design 2.2.1 Individual SC-SOFC design The experimental platform for studying individual micro SC-SOFCs is shown in Figure 14. These individual cells are created to test a wide range of parameters in an attempt to isolate the fundamental electrochemical processes. The control cell, which every test fuel cell in this study is compared to, consists of two equal sized electrodes (5 by 100 microns) with a separation distance of 5microns. Each pair of electrodes is placed upon its own individual electrolyte layer that borders the pair of electrodes by 5microns. Sets of experiments were performed on the control fuel cell where only one of the following parameters were changed each time: anode width, cathode width, gap separating the electrodes, lengths of both electrodes (varied simultaneously), and widths of both electrodes (varied simultaneously). 37 . ... For every variable that is changed, a change in performance is expected to occur. By changing the gap separating the electrodes, the relationship between gap distance and electrolyte performance can be studied, which can help determine the sensitivity of performance due to electrode alignment and positioning. Individually changing the width of the anode or cathode can provide insight into several design conditions for micro SCSOFCs, such as what electrode impacts the rate limiting reaction, and if increasing the size of the rate limited electrode could improve fuel cell performance. Changing the electrodes length and width can help determine if the reactions are dependent on the electrodes area or triple phase boundary (electrode perimeter, i.e. the boundary between the electrode, electrolyte, and chemical species). Anode Length Anode Width 4 hoarder Cathode Width K-Cathode Length b Figure 14: Schematic for individual micro SC-SOFC test cells. 2.2.2 Stacked Micro SC-SOFC Design The stacked micro SC-SOFC design shown in Figure 15 uses the individual control micro SC-SOFC discussed in Section 2.2.1 as the base fuel cell. Experiments were done with the stacks varying three variables: number of cells in a stack, the length of each cell in the stack, and the number of interconnections between fuel cells (either one interconnect between fuel cells, or one interconnect every 25 microns). Experiments with changing the number of fuel cells in a stack are used to validate the ability of micro SC-SOFCs stack in series to increase its voltage linearly. To understand how a micro SC-SOFCs stacks total current and current density change with size, several different stacks are fabricated with fuel cells of different lengths. Experiments with changing the amount of interconnects used for stacking micro-SC-SOFCs are used to 38 determine if a single interconnect is sufficient (i.e., does not limit current), or if several interconnects are needed to fully utilize a series stack of fuel cells. 0 0 0 S Stack * Length Slogi Interconnect mkro SC-SOFC . _ _ ~c colic Stack Longth tultiple lcttecolntt micro SC-sOtC Stok Dwft b a Figure 15: Schematic for planar single (a) or multiple (b) interconnect micro SC-SOFC stack. 2.3 SC-SOFC Fabrication In these micro SC-SOFCs, 8mol% yttria-stabilized zirconia (YSZ) (Praxair Specialty Ceramics, USA) was used for the electrolyte; platinum (Pt) and gold (Au) were used for the electrodes. Figure 16 depicts the fabrication steps used to produce micro-sized SCSOFCs: (1) a 4 inch silicon wafer is used as the SC-SOFC support; (2) spin-coating of negative photoresist (Clariant, Switzerland) is applied; (3) wafer is covered by photomask with electrolyte features, then exposed; (4) YSZ is deposited by sputtering for 2 hours at 100W power and 60:8 ratio of Ar:0 2; (5) excess YSZ is lifted off by removing the resist with a photoresist stripper, leaving the desired electrolyte features; (6) sample is re-coated with negative photoresist; (7) wafer is covered by the photo-mask with the first electrode features, then exposed; (8) Au is deposited by sputtering for 25 minutes at 100W power and pure Ar environment; (9) excess Au is lifted off by removing the resist 39 with a photoresist stripper, leaving the desired Au electrode features; (10) sample is recoated with negative photoresist; (11) wafer is covered by the photo-mask with the second electrode features, then exposed; (12) Pt is deposited by sputtering for 25 minutes at 100W power and pure Ar environment; (13) excess Pt is lifted off by removing the resist with a photoresist stripper, leaving the desired Pt electrode features, and completing the micro SC-SOFC. The YSZ, Au, and Pt thicknesses were all found to be approximately 200nanometers using AFM measurements. Deposit 1st Electrode Si Wafer -0 Lift off NCoat w/ 2resist UV expose ~O & Develop Coat w/ resist Deposit P0 Electrolyte D UV expose & Develop Lift off Deposit 2nd Electrode Coat w/ resist UVexpose - &Develop Si Wafer Resist Electrolyte 1st Electrode Figure 16: Steps for micro SC-SOFC fabrication. 40 Lift off 2nd Electrode 2.4 Experimental Setup 2.4.1 Testing Conditions and Apparatus Samples were placed inside a microprobe station (Karl Suss, Germany) equipped with an optical microscope (Mitutoyo, Japan) and a temperature controlled stage (Linkam TS1500, UK), with a custom-sealed gas chamber enclosure. A Princeton Applied Research Potentiostat 263A was used to measure OCV and collect current verse voltage data. OCV measurements were taken between 400 and 900*C in increments of 100*C and the data were averaged over a 120-second span. A mixture of methane, oxygen, and nitrogen, in a volume ratio of 1:1:5 or 2:1:5 and at a flow rate of 140 mL/min or 160mL/min respectively, was supplied to the chamber. 2.4.2 Current-Voltage Data Collection A standard method for monitoring the operation of a fuel cell is to create current-voltage (IV) plots, as shown in Figure 17. A preferred energy conversion device would be able to provide a constant voltage for any current drawn from the cell. However activation losses, ohmic resistances and mass transport limitations all contribute to an overall reduction in performance. This voltage loss relationship is shown in Eq. 4. 1.2 l 1.0 -- - - - - - - - - - - - - - --.-Vvotg -f Graph is fairly linear 0.8 0.6 V small initial tall in voltage, and open circuit voltage is very close to theoretical value = 0.4 - Voltage begins to fall faster at higher currents 0 0 200 400 600 800 1000 2 Current density (mA cm- ) Figure 17: Typical IV graph for SOFC operating at 800*C(5). V = Vocy - A Vactivaionoss - (Ranode + Rcathode + Reecroyte )I - AV.sstransport Eq 4 41 SOFCs have very little activation losses due to the high operating temperatures. In wellfueled systems that do not demand high current densities, mass transport losses can also be neglected. Therefore voltage will be proportionally dependent upon the combined resistances of the anode, cathode, and electrolyte. To help determine which resistance has the largest contribution, AC impedance spectroscopy can be performed to determine the extract electrolyte and electrode resistances. Once these resistances are determined, they can be used in Eq. 4 to provide insight to the fuel cell performance. 2.4.3 AC Impedance Spectroscopy AC Impedance Spectroscopy is a common analytical tool for evaluating solid state devices(46). By modeling the fuel cell as a simple network of resistors and capacitors, it becomes a customary electrical circuit. An alternating voltage at a certain frequency is applied to the system, the current output is measured, and an impedance is calculated. As the voltage frequency is changed, a spectrum of impedances can be created which can be correlated to the device's operation. Figure 18a shows a simplified circuit diagram for a fuel cell and Figure 18b shows an ideal ac impedance plot(46). From the AC impedance plot, Figure 18b, R, represents the resistance of the fuel cell's electrolyte layer. Resistor and capacitor, R2 and C2, correlate to the electrode's resistance, which, when plotted with ac impedance, creates a semi-circle graph where the diameter of the semi-circle is the resistance associated with the electrodes. All R" (electrolyte resistance) and R 2 (electrodes/fuel cell resistance) are extracted from the collected data in order to compare test results. AC impedance spectroscopy measurements were conducted on a Solartran 1260 frequency response analyzer connected to a Solartron 1296 dielectric interface in the frequency range of -OMHz to -100 Hz using an ac voltage amplitude of 30-5OmV with zero dc bias. The integration time varied between 10-30seconds depending on the clarity of the data obtained. Each data experiment was run 2-3 times and averaged together to account for measuring variability. 42 a lectrodes/Fuel Cell Resistance Electrolyte Resistance -Im(Z) (RIO-R 2) Re(Z) b where R. and C. represent the effects diagram circuit cell Figure 18: Simplified fuel imposed by the electrolyte and R2 and C2 represent the electrode effects (a). Ideal ac impedance plot for the circuit above (b) (46). The main experiments conducted evaluate the resistance characteristic for different electrode configurations. The four main electrode configurations evaluated are all the combinations of gold and platinum electrodes with widths of 5micron and 15micron. Table 4 lays out the main types of cells tested and the nomenclature for the collected data. Table 4: AC Impedance experimental nomenclature Nomenclature Width Electrode 'Pt Au' (micron) Material Cell 1 Platinum Gold 5 5 '5 5' Cell 2 Platinum Gold 5 15 '5_15' Cell 3 Platinum Gold 15 5 '15_5' - Cell 4 Platinum Gold 15 15 '15 15' 43 2.4.4 Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) Images of the tested samples were obtained by scanning electron microscopy (SEM) on a Jeol 6320 operating at an accelerating voltage of 15kV for imaging and x-ray energy dispersive spectroscopy (EDS). Images revealing the molecular structure for certain samples were achieved by transmission electron microscopy (TEM) on a Jeol 201 Of operating at an accelerating voltage of 200kV. 2.4.5 X-ray Diffraction Thin film X-ray diffraction was performed to help confirm the initial molecular composition of the samples before and after experimentation to determine if any changes occurred in the materials structure or composition after testing. Thin film X-ray diffraction patterns were collected by Bruker D8 DISCOVER equipped with a twodimensional general area-detector diffraction system (GADDS). The K alpha radiation of 1.5418 angstroms was generated in a Cu tube at 40 kV and 40 mA. The X-ray beam was focused using a collimator with a 0.5 mm beam diameter. The angle between a thin film sample and the incident X-ray beam (omega angle) was set to 10 degrees. 44 3.0 Results 3.1 Individual Micro SC-SOFC Operation at Various Temperatures Operation characteristics for individual micro SC-SOFCs at various temperatures are shown in Figures 19a-c. The micro SC-SOFC gold and platinum electrodes in this experiment are 15microns by 400microns separated by a 5micron gap. The fuel cell was placed into a custom sealed gas chamber. The chamber was purged for 60 minutes with the operating gas composition of 1:1:5 of methane:oxygen:nitrogen with a total flow rate of 140ccm. Once the purge was complete, the temperature of the fuel cell was raised to 400*C at a rate of 10*C per minute. After holding the temperature at 400*C for 15 minutes, microprobes were put in contact with each electrode to monitor the OCV for three minutes and to collect the current-voltage data. After repeating the data collection process at least once, the probe tips were lifted off the sample, and the chamber temperature was increased by an additional 1 00*C at a rate of 10*C per minute. Once the new temperature was reached, the data collection process was repeated until data had been collected from the fuel cell up to a chamber temperature of 900*C. For the individual micro SC-SOFCs, as the fuel cell temperature increases, the OCV goes from a negative to a positive value between 500*C and 600'C. Once the voltage becomes positive, a stable voltage of ~0.4V is maintained from 700*C to 900*C, Figure 19a. Current is pulled from the fuel cell only when the temperature is above 600*C. Below 600*C either no current can be measured from the fuel cell or the value results from the instrumentation. Above 600'C, as temperature increases, the amount of current drawn from the cell increases, reaching current density of up to 500 A/cm 2 when the electrodes are shorted, Figure 19b. Conversion of the current-voltage data into power density values shows a maximum power density of 27 tW/cm 2 when operating at 900'C, Figure 19c. 45 Open Circuit Voltage at various SC-SOFC Temperatures 0.5 0.4 0.3 > 0.2 0.1 f -0.10 -0.2 -0.2 500 400 900 800 700 600 Temperature (C) a Voltage - Current Density at Temperature 400-900C 0.5 0.40.3 400C *500C 600C 70C 9 02 0.1 ~00 800C 900C .0.2-0.3 -0.4 100 0 200 300 400 500 600 Current (uA/cmA2) b Power Density- Current Density at Temperature 400-900C 30 25 400C a 500C 600C 700C 800C < 20 .. is 10 900C 5 0 - 0 100 200 300 Current (uA/cmA2) 400 500 600 C Figure 19: An individual micro SC-SOFC with 15micron by 400micron Pt and Au electrodes in 1:1:5 methane:oxygen:nitrogen fuel ratio, OCV at various temperatures (a), current-voltage at various temperatures (b), current density-power density at various temperatures (c), 46 3.2 Micro SC-SOFC Electrode Changes at Various Temperatures and Gas Compositions During the initial micro SC-SOFC experiments, some of the gold and platinum electrodes had transformed from solid uniform layers to porous or rough edged layers. In an attempt to understand this observation, various samples were placed into the heated gas chamber and exposed to different gas environments: pure methane, pure oxygen, pure nitrogen, and a 1:1:5 mixture of all methane:oxygen:nitrogen. The samples were purged with the selected gas mixture for one hour, and then heated to 400*C at a rate of 10*C per minute. Keeping the temperature at 400C, digital photos of the sample were taken through the gas chamber in order to capture the effects temperature and the gas composition had on individual and stacked micro SC-SOFCs. Once the data were collected and documented at 400*C, the temperature was increased 50C at a rate of 15C per minute. Upon reaching the new temperature, several pictures were quickly taken. The fuel cell was kept at the new temperature for 15 minutes, and additional pictures were taken to document any quasi steady state changes that may have occurred. This process was repeated up to the final resting temperature of 850*C, and held at that temperature for an additional 60 minutes to determine if any additional transformation of the electrodes would occur. Figures 20a-d shows the initial condition for each sample at 400*C. Figures 21a-d shows the final condition for each sample after being held at 850C for over an hour. Summaries of the key changes that occurred are highlighted in Table 5. This experiment was conducted in an attempt to isolate the effects that are caused by chemical reactions between the gas species and the electrodes, phase transformation of the electrodes, temperature, or possible electrochemical reactions occurring in the fuel cell. 47 ah d c Figure 20: Au and Pt electrodes on YSZ electrolyte upon a silicon substrate at 400*C exposed to various gas compositions: Nitrogen (a), Oxygen (b), Methane (c), Methane:Oxygen:Nitrogen in a 1:1:5 volume flow rate ratio(d). 48 9 iib C d Figure 21: Au and Pt electrodes on YSZ electrolyte upon a silicon substrate at 850C exposed to various gas compositions: Nitrogen (a), Oxygen (b), Methane (c), Methane:Oxygen:Nitrogen in a 1:1:5 volume flow rate ratio(d). 49 400 700 Table 5: micro SC-SOFC electrode transformation summary All CH4 N2 02 Onset of Pt Au defects over Pt defects on all Pt defects on all cells defects in Si and on Series cells* Series cells cells 750 Slight increase in Pt defects Slight increase in Au defects Onset of Pt 800 Pt defects increase in Series cells, Onset of Pt defects in Single cells Pt defects increase on all cells, but minimally Increase in Pt defects, onset of Au defects* Increase in Pt defects Slight increase of defects in both Au an Pt Increase in Au defects* Pt uniformly deformed in all cells Slight increase of Au defects, a few large Pt defects Au substantially degraded, increased Pt defects, but not as severe as No change, Pt uniformly deformed defect 850 Au* Summary Slight Pt defects, no affect on Au Overall minimal effects, smaller uniform deformation in Au, and a few Pt degrades, then Au, a switch occurs* Pt uniformly became porous, Au unaffected I large Pt defects this is the most severe results observed at this test condition, but the results are inconsistent from test to test, it is unclear at this time what effects influence reproducibility * When the micro SC-SOFCs are exposed to nitrogen or oxygen, Figure 21a and Figure 21b respectively, there is very little change to the gold and platinum electrodes. Some small features develop primarily over the silicon, but several small features also develop on the electrodes. When exposed to methane, Figure 21c, gold starts to transform at lower temperatures followed by the platinum transformation at higher temperatures (note: these are the most severe results observed at this test condition, but the results are inconsistent. It is unclear at this time what effects influence the experiment's reproducibility). When exposed to a mixture of gases, Figure 21d, gold is unaffected 50 while the platinum electrodes of the single cells deform and develop pores at the surface, and these for the stacked cells completely degrade off of the surface of the YSZ. 3.3 Single Micro SC-SOFC Performance To further understand how these micro SC-SOFC perform, additional OCV and currentvoltage plots were obtained for cells at 900*C exposed to methane:oxygen:nitrogen in a 1:1:5 ratio and a cumulative flow rate of 140ccm. The fuel cells tested all had platinum and gold electrodes that were 5microns in width and lengths varying from 1 00microns to 400microns. The other variable evaluated is the gap between electrodes. The data shown in Figure 22 has a gap distance of 15microns, while Figure 23 shows the data for a gap distance of 5microns. For individual micro SC-SOFCs with 5micron wide platinum and gold electrodes separated by a 5micron or 15micron gap and exposed to methane:oxygen:nitrogen in a 1:1:5 fuel ratio at 900*C, a clear trend is evident between the fuel cell area and performance; holding the electrode width constant at 5microns and changing the electrode length results in lager area and higher power and current output. The OCVs for both types of cells, Figure 22a and Figure 23a, are both slightly lower but comparable to earlier results shown in Figure 19. This slight reduction in OCV is possibly attributed to the thin electrodes being more affected by gas species mixing than the larger 15micron wide electrodes. Mixing of the gas species may lower the partial pressure and hence reduce the OCV as shown in Eq. 1. At the electrode gap distance of 15microns, the maximum current and power are approximately 9.5nA and 0.95nW when the electrodes are at the largest size of 400microns in length. Similarly, at the gap distance of 5 micron, the peak current and power are 15nA and 1.1 nW. 51 ocV @ 900C Electrolyte: YSZ Electrodes: Au & Pt Smicron, XXXmicron length with 15micron gap Gas: CH4:02:N2 [20:20:100 ccm] 0.4 0.3 0.2 01 0A 0 - -- - - ------- -~ 400micron 200micron 100micron fuel Cell Length a IV © 900C Electrolyte: YSZ Electrodes: Au & Pt 5micron, XXXmicron length with 15micron gap Gas: CH4:02:N2 [20:20:100 ccm] 0.4 O100micron 0.3 + 0 0.2 + 0.1 trial 1 a 100micron trial 2 x 100micron trial 3 200micron trial 1 200micon trial 2 200micron trial 3 400micron trial 1 400micron trial 2 i, U 2 0 4 12 10 8 6 Currant (nA) b Power 0 900C Electrolyte: YSZ Electrodes: Au & Pt Smicron, XXXmicron length with Gas: CH4:02:N2 (20:20:100 ccm] 15micron gap 100micron trial 1 * 0.8 0.6 trial 3 trial 1 troal 2 trial 3 400micror trial 1 400micron trial 2 0A6 0.2 0 100micror trial 2 100micron 200micron 200micron 200micron - 2 8 6 10 12 Currant (nA) C Figure 22: Individual micro SC-SOFC with 5micron wide Pt and Au electrodes separated by a gap distance of 15micron and exposed to methane:oxygen:nitrogen in a 1:1:5 fuel ratio at 900 0 C, average OCV as a function of fuel cell area holding the electrode width constant at 5microns and varying the electrodes length (a) currentvoltage (b) current-power (c). 52 Electrodes: Au & Pt ocV a 900C Electrolyte: YSZ 5mlcron, XXXmicron length with Gas: E > Smicron gap C1H4:02:N2 (20:20:100 ccm] 2 fuel ...... .............. .. ..... .. ... Coli length a IV 0 900C Electrolyte: YSZ Electrodes: Au & Pt 5micron, XXXmicron length with 5micron gap Gas: CH4:02:N2 [20:20:100 ccm] 0.4 0.3 100micron 7 0.2 V 9 trial 1 a 1Omicron trial 2 100micron trial 3 9 400micron trial 1 9 > 400micron trial 2 0 9 6 3 0 15 12 Current (nA) b Power 0 900C Electrolyte: YSZ Electrodes: Au & Pt Smicron, XXXmicron length with Smicron gap Gas: CH4:02:N2 [20:20:100 ccm] 1.2 ,08 Il00micron trial 100micron trial K400micron trial 400micron trial 0.6 0.4 0.2 2 3 I 2 -- 0 0 2 10 4 12 14 Current (nA) C Figure 23: Individual micro SC-SOFC with 5micron wide Pt and Au electrodes separated by a gap distance of 5micron and exposed to methane:oxygen:nitrogen in a 1:1:5 fuel ratio at 900 0 C, average OCV as a function of fuel cell area holding the electrode width constant at 5microns and varying the electrodes length (a) currentvoltage (b) current-power (c). 53 3.4 AC Impedance Spectroscopy: Further Micro SC-SOFC Characterization and Isolation of Electrode and Electrolyte Effects AC impedance spectroscopy is conducted to help analyze the characteristics for micro SC-SOFCs. In an attempt to obtain results uninfluenced by corrosion, lower temperatures of 600'C to 700'C were used. However, due to the long time it takes to run AC impedance, the platinum electrode in a mixed gas mixture would still transform even at the low temperatures. Thus, the fuel cells were held at the desired temperature to allow for the electrode transformation to reach steady state in order to avoid measuring dynamic changes to the electrode. The four main micro SC-SOFCs studied are outlined in Table 4. The electrode and electrolyte resistances are shown in Figure 24a and Figure 24b respectively. These figures show the results of two trials conducted on two different fuel cells. Each trial was conducted with a 400micron long micro SC-SOFC at 700C with a methane:oxygen:nitrogen ratio of 1:1:5 and a cumulative flow rate of 140ccm. Repeating this experiment on two different samples helps to establish a baseline for the data's repeatability among different tests. While the two different trials do not completely overlap, they are within experimental error. The electrolyte resistance is within 1Mohm for each trial as expected since the electrolyte resistance should be the same for each cell. The electrolyte resistance shown in Figure 24b is relatively unchanged and within the same tolerance of 1 Mohm, indicating that the electrolyte resistance is fairly constant from one cell configuration to another. The average resistance, however, does change with respect to the electrode configuration, Figure 24a. The overall resistance decreases as the width of one electrode is increased. However when the widths of both electrodes increase by the same amount, the resistance increases, contrary to what's expected. These experiments seem to indicate that creating electrodes that are asymmetrical in size benefit the fuel cells operation. This observation has been reproduced with the two trials 54 above, however the physical origin for this observation is not understood and additional tests will be conducted to try and understand these results. Electrode Resistance for 400micron Long SC-SOFC CH4:02:N2=> 1:1:5 5 .10E Electrolyte Resistance for 400micron Long SC-SOFC CH4:02:N2=> 1;1:5 1.00E+08 08 S1E00++07 1.00E+06 i 3.10E+08 2,10E+08 1.00E+05 E 1 105-08 100E+07 - Pt 5_A 5 -*- -- ~ 1: 1 700C trial 1 1: @C70CC trial 2 E+04 ~, Pt 5_ Au 15 Pt 15 Au 5 Pt 15_Au Electrode Type and Width (microns) 15 - Pt 5 A 5 -- -1- 5 Pt 5 Au 15 Pt 15 Au Electrode Type and Width (microns) Pt 15_Au 15 1:1 a 700C trial 1 1@ 700C trial2 a b Figure 24: Two separate trials for various 'PtAu' width electrodes that are 400micron long exposed to 1:1:5 ratio of methane:oxygen:nitrogen at 700'C, average electrode resistances (a), average electrolyte resistances (b). In an attempt to improve the data collection using AC impedance, the methane to oxygen ratio was increased to 2:1, resulting in a cumulative flow rate of 160ccm when combined with 5 parts nitrogen. Using this fuel mixture, the four main fuel cells as mentioned in Table 4 were tested at 600 0 C and 700 0 C, the electrode and electrolyte resistances are shown in Figure 25a and Figure 25b respectively. attempted. Data collection at 800 0 C was However, at this temperature, a new form of platinum transformation occurred, tainting the AC impedance measurements. This new transformation effect will be discussed in Section 4.3. In this mixture at 600*C and 700 0 C, the average resistance has the same trend as shown in Figure 25a. With these parameters, the overall resistance follows the expected trend, and the lowest resistance is achieved when both electrodes are the largest. The relationship between electrolyte resistance and temperature is also as expected for each temperature tested, Figure 24b: the higher the temperature, the lower the electrolyte resistance. 55 Electrolyte Resistance for 400micron Long SC-SOFC Electrode Resistance for 400micron Long SC-SOFC SOE+08 7 - ----- 1.OE+08 4.OE+08 E .C E C 3.OE+08 2,OE+08 :1. - 1.OE+06 0E+08 1.0E+06 0 1.OE+07 - 1.E+05 Pt 15_Au 5 Pt 15_Au 15 Pt 5 Au 15 Pt S-A 5 Electrode Type and Width (microns) - -...... - -..... Pt 5_ Au 15 Pt 15Au 5 Pt 15-Au 15 Electrode Type and Width (microns) -- Pt 5_A 5 2:1 of CH402 @ 600C -0 2:1 of CH4:02 ( 700C of CH4:02 @ 600C -2:1 4- 2:1 CH4:02 @ 700C a b Figure 25: Various 'PtAu' width electrodes that are 400micron in length exposed to 2:1:5 ratio of methane:oxygen:nitrogen at 600'C and 700*C, average electrode resistances (a), average electrolyte resistances (b). In the 2:1 methane to oxygen environment at 600*C, the electrode and electrolyte resistances are respectively shown in Figure 26a and Figure 26b for three different length of micro SC-SOFCs (100micron, 200micron and 400micron). As length increase, the average resistance and electrolyte resistance decreases, as expected. Additionally, the electrolyte resistance is virtually constant for all of the fuel cell configurations that have the same length. The final AC impedance test is used to analyze the effect of electrode-electrode gap distance on performance. 5micron by 400micron gold and platinum electrodes separated by 5, 10, and 15micron gaps were exposed to 2:1 methane to oxygen, at 600'C. The effect of electrode gap separation on electrode and electrolyte resistances are shown in Figure 27a and Figure 27b, respectively. An increase in gap distance increases the electrolyte resistance and the overall resistance. 56 Micro SC-SOFC Overall Resistance CH4:02:N2= >40:20:100 @600C - 5.OE+09 0 -- 4.OE+09 3.OE+09 2.O E+09 1.QE+09 - - - - -- 1 - 1.OE+07 Pt5_A 5 -4- 400micron -U- 100micron Ptl15_Au 5 PtS5_ Aul15 and Width (microns) Electrode Type Ptl15_Au 15 -k- 25micron a Electrolyte Resistance CH4:02:N2=>40:20:100 @600C 1.OE+08 E o 1.0E+07 1.OE+06 1.OE+05 Pt 5_A 5 Pt 5 Au 15 Pt (5Au5 Pt 15_Au 15 Electrode Type and Width (microns) -*- 400micron -U- 100micron -1r- 25micron b Figure 26: Various 'Pt Au' width electrodes that are 100micron, 200micron and 400micron in length exposed to 2:1:5 ratio of methane:oxygen:nitrogen at 600 0 C, average electrode resistances (a), average electrolyte resistances (b). 57 Electrode Resistance for 400micron Long, 'x' Electrode Gap Distance CH4:02:N2=>40:20:100 @600C 3.50E+08 ~~~~ - ~~.. _-......7 ~7~.~ ............. ----.. ..... -_ - _ .- - --. - .......... ... -.. ---.. - -... ------- 3.00E+08 IA 0 . ... ..-.. 2.50E+08 ..... .... - ... --... -- ..... ........ ...... .... .-.. ...... 2.OOE+08 C 1.50E+08 1.OOE+08 5.00E+07 5 micron 10 micron Electrode-Electrode Gap Distance 15 micron a Electrolyte Resistance for 400micron Long, x' Electrode Gap Distance CH4:02:N2=>40:20:100 @600C 9.OOE+06 8.00E+06 ' E 7.OOE+06 6.00E+06 C 5.00E+06 4.00E+06 3.00E+06 15 micro 2.00E+06 1.00E+06 10 micron 5 micron 15 micron Electrode-Electrode Gap Distance b Figure 27: 5micron wide Pt and Au electrodes separated by a gap distance of 5micron, 0 10micron, and 15micron exposed to 2:1:5 ratio of methane:oxygen:nitrogen at 600 C, average electrode resistances (a), average electrolyte resistances (b). 58 3.5 Stacked SC-SOFC Data One of the ultimate objectives of this research is to develop high voltage micro SC-SOFC stacks. Several design concepts were proposed in Section 2.2.2 and later fabricated and tested using 1:1 and 2:1 methane to oxygen. However, these stacked fuel cells were the first to show platinum transformation. This transformation happened so quickly that it rapidly severed the series connections between the stacked fuel cells. As a result, the multi-connection stacked fuel cells, as depicted in Figure 15b, degraded quickly, making it impossible to obtain a stack OCV. Therefore, to study the effects of serial stacking, stacked micro SC-SOFCs with a single interconnect were exposed to a 2:1:5 ratio of methane:oxygen:nitrogen at 600*C. Figure 28 shows the data of 10 cells stacked in series where each cell is composed of 400micron by 5micron wide electrodes separated by a 5micron gap. After obtaining the overall stack OCV, the voltage between the first 8, 6, 4, 2, and 1 cells was obtained to determine the voltage change along the fuel cell stack. The peak OCV obtained with all 10 cells stacked in series was 3.3 volts. OCV of micro SC-SOFC Stack 3.5 S 3 2.5 * 10 cells in series * 8 cells in series 6 cells in series * 4 cells in series * 2 cells in series 1 single cell 22 5 1.5 1 0.5 0 100 150 200 Time (s) 250 300 Figure 28: 10 cells connected in series measuring the overall OCV as well as the OCV between the first 8, 6, 4, 2 and 1 cells. Each individual cell is 400micron long with 5micron wide electrodes, exposed to 2:1:5 methane:oxygen:nitrogen at 600*C. 59 60 4.0 Discussion 4.1 OCV verse Temperature: Electrode Catalytic Activity Reversal The influence of temperature on the characteristics of noble metal micro SC-SOFC operation shows rather interesting behavior. As temperature increases, the OCV reverses direction, going from a negative voltage at 400*C to a positive voltage at 600'C and above, as shown in Figure 19a. Knowing the composition of the gas exposed to the fuel cell may help determine which electrochemical reactions may be occurring at the different temperature regimes. Figure 29 shows the thermodynamic equilibrium states for a 1:1:5 ratio of methane:oxygen:nitrogen over the temperature range of 400*C1 000*C, and is similar to those found in literature (47). The main chemical species in the chamber exposed to the fuel cell are hydrogen, carbon dioxide, carbon monoxide, water, methane, and nitrogen. Table 6 gives the molar fractions of these chemical species at 500'C and 800*C. Oxygen is not listed since it is present at a very low concentration, but its presence facilitates the electrochemical reaction at the cathode. 0 12 C 0.10 S0 08 2 OC H2 0 C-- E- 0,04 02 S004 000 400 Equilibrium Mole traction H Equilibrium Mole traction CO Equilibrium Mole traction CO Equilibrium Mole fraction HH4 Equilibrium Mole traction H2 1000 800 900 600 700 500 Temperature C1 Equilibrium Clusteri (Cl) (C) Figure 29: CHEMKIN thermodynamic equilibrium simulation over various temperatures for a 1:1:5 ratio of methane:oxygen:nitrogen. 61 Table 6: Thermodynamic equilibrium of gas species in the chamber with initial starting ratio of 1:1:5 methane:oxygen:nitrogen for 500'C and 800*C. Species molar fraction at 'X' temperature 800 0 C 500 0 C 0.12 0.06 H2 0.04 0.05 CO 2 0.055 0.01 CO 0.055 0.05 H2 0 <0.001 0.025 CH 4 0.73 0.805 N2 The potentiostat is connected to the fuel cell via the platinum anode and the gold cathode. When the fuel cell temperature is below 600'C, the OCV is measured and, surprisingly, a negative voltage is observed. However, when the fuel cell is operating above 700*C, an OCV of approximately 0.35V is observed. Although 0.35V is not particularly close to the theoretical OCV value of 0.98V, it closely correlates with reported results for microand standard-sized single chamber fuel cells (8, 23, 28). Previous studies have attributed the discrepancy between the theoretical OCV value and the lesser observed OCV value to one of two possibilities: product gas species mixing with reactant gas species, thereby reducing electrode reactivity, or poor catalytic electrode materials (8, 17, 23, 42). At higher temperatures the fuel cell obtains an OCV similar to reported values, confirming that the platinum anode is oxidizing hydrogen, while the gold cathode reduces oxygen. In addition to hydrogen and hydrocarbon fuels, SOFCs are known to be able to use carbon monoxide as a fuel. However, it is known that in the presence of water, carbon monoxide will undergo a water-gas shift reaction to form hydrogen and carbon dioxide. This hydrogen can then be reutilized in the electrochemical reaction (48). The results shown above indicate the possibility that at 800 C, when there is an almost 1:1 ratio of water to carbon monoxide, the carbon monoxide will undergo a water-gas shift reaction to form hydrogen and carbon dioxide. This water-gas shift reaction is beneficial in several ways: it creates more hydrogen for an electrochemical reaction, and it transforms the carbon monoxide into species that do not reduce the activity of platinum towards 62 27t hydrogen oxidation (48). A schematic for the anode and cathode reaction at 700*C and higher the can be seen in Figure 30. ee Co 117 Ii() C( O7 CO) H' H2 C- -- H2 H2 + C0 2 H2 O *co 4GH,2 Cathode 02+ 4e- -> 202- Anode +700 0 C 2H 2 +02 -+ 2H 20 + 202- -> 2H20+ 4e- Figure 30: Reaction mechanisms for micro SC-SOFC w/ gold and platinum electrodes in a 1:1:5 methane:oxygen:nitrogen mixture at >700'C include water-gas shift reaction of CO, H2 is oxidized on the platinum electrode, while 02 is reduced on the Au electrode. In the low temperature regime, the proposed electrochemical reaction is more complicated. It would appear that the same process that occurs in the high temperature regime could happen in the low temperature regime, but there are a few differences. It is known that nitrogen and carbon dioxide should not be reactive and their only influence on the performance of the fuel cell is reducing the partial pressure of the reacting fuels. The methane that has not already decomposed will react with the water to form more hydrogen and carbon dioxide. Once methane is reformed there is significantly less water available at 500'C than at 800*C remaining to drive the water-gas shift reaction to push carbon monoxide towards creating carbon dioxide. The presence of the remaining carbon monoxide is the key property in this process at temperatures below 600'C. Carbon monoxide is known to coat platinum easily, which then renders it inactive as a hydrogen catalyst. This event is often referred to as platinum carbon monoxide poisoning, which can occur for low temperature polymer exchange membrane fuel cells (49). The reason 63 for platinum carbon monoxide poisoning is due to the similarity between the binding energy of carbon monoxide and its activation energy with platinum. Figure 31 shows how carbon monoxide is converted into carbon dioxide on a platinum surface over a range of temperatures. Platinum can only react with another species if carbon monoxide is desorbed from its surface or if the carbon monoxide undergoes a water-gas shift reaction to form carbon dioxide. If there is not enough water for a water-gas shift reaction to occur, the binding and activation energy levels are too similar for carbon monoxide to be desorbed from the platinum surface, and the rate of carbon dioxide formation is very slow (50). Thus, at lower temperatures, the platinum electrode is very inactive with regards to reacting with hydrogen or another species, due to carbon monoxide acting as a catalytic barrier. 1000 Pco. I$ S 10 1 \~.--5% IriSiO2 W o e to(? 0.1t100K0/ s $%Pd 0) 1 es (K ) 0) . 0.01 0.001 1.4 _____ 1.8 1.8 2.0 2.2 2.4 2.8 1 1000/T (K- ) Figure 31: Platinum, palladium and iridium CO to CO 2 formation rates as a function of inverse temperature for single crystal supported catalysts (50). Interestingly, gold can oxidize carbon monoxide, even in a mixed gas environment. Gold is very inactive towards methane, hydrogen, and nitrogen, but studies have shown that gold nanoparticles can oxidize carbon monoxide (51, 52). Therefore, while carbon monoxide reduces the performance of the platinum, gold has the ability to oxidize carbon monoxide to form carbon dioxide, thereby making gold act as the anode while platinum reduces oxygen to complete the reaction. This switch in activity would explain why a 64 -7. M-TT - . , _ 1.-- __ 2 __- ____ __ , I" ____ A schematic for the proposed negative voltage is observed at lower temperatures. reactions at lower temperatures is shown in Figure 32. e-- (I'I11, 0I CWH0 + 2H 2 0 C114 I II 4H2 + C C1111 co 2CHA 2CO2 402 4H Anode 2CO + 202 -> 2CO, + 4e <600C 2CO+0 2 2+ Cathode 4e~ -> 202 -> 2CO 2 Figure 32: Reaction mechanisms for micro SC-SOFC w/ Au and Pt electrodes in a 1:1:5 methane:oxygen:nitrogen mixture at <600*C include water-gas shift reaction for the methane, carbon monoxide blocks the platinum electrode which promotes oxygen reduction, while carbon monoxide is oxidized on the gold electrode. The role of carbon monoxide at lower temperatures also explains why no current can be drawn from the fuel cell below 600*C. It is difficult for carbon monoxide to be removed from the surface of the platinum electrode, thereby making it the rate limiting electrode. There are several theories to explain why the operating OCV above 700*C is ~0.4 V instead of the theoretical value of 0.98V. One proposed theory is that when the electrodes are too close to each other, the products of each electrode reaction mix and dilute the reactants for the opposite electrode. This mixing reduces the partial pressures of the fuel cell's reactants, which in turn reduces the fuel cells performance (44). The effect on OCV of increasing the distance between the anode and cathode has been studied, and shows that the OCV becomes smaller and less stable as the distance between the electrodes gets smaller (28). Both of these studies observed OCVs around O.4V with 65 the distance between electrodes being less than half a millimeter. The other theory is that the low OCV is directly correlated with the material properties of the electrodes. Platinum is susceptible to coking, which can reduce the voltage over time. Additionally, platinum and gold have high overpotentials associated with their ability to adsorb and/or ionize oxygen (23). There is reasonable evidence to show that the low OCV observed in these noble metal micro SC-SOFCs is due to the effects of mixing and high overpotentials. The effect caused by having the electrodes close together makes these cells very susceptible to product and reactant mixing. Without using more selective materials, this geometric constraint is certainly a factor that affects the observed OCV. The large overpotentials of platinum and gold further support the observation that these materials are poor selective catalysts and can also explain why the observed OCV is smaller than the theoretical value. Given these constraints, the micro SC-SOFCs created have met or surpassed reported OCV values for platinum and gold electrodes as well as for other micro SCSOFCs (23, 28, 44). Future studies will utilize alternative materials to help improve selectivity and reduce overpotentials. 4.2 Electrode Transformation When the micro SC-SOFCs in a mixed gas environment of methane, oxygen and nitrogen are operating at temperatures above 600'C, it is apparent that the electrodes undergo physical transformation as previously shown in Figure 21. SEM pictures of this transformation demonstrate the changes to the platinum electrode on an individual micro SC-SOFC, Figure 33a, a stack of 5 cells in series with a single interconnect Figure 33b, several smaller 5 cell stacks with a single interconnect, Figure 33c, and a 5 cell stack with multi-interconnects, Figure 33d. Looking at the individual cells, the platinum appears to become porous, but the electrodes do not appear to be changing in size. This observation eliminates the possibility for the 66 deposition of an introduced substance such as carbon or the formation of new compounds such as PtN. In single cells, the platinum transforms, but can still be seen on the electrolyte; in series stacks, however, the transformation occurs so rapidly that in most cases, the electrodes disappear. This may be a result of platinum breakdown or its diffusion into the electrolyte. Ium 1plm b a 4 Pt A Al L 100pm -4P_ 1O0ijm c d Figure 33: SEM images documenting the transformation of Pt-YSZ-Au micro SC-SOFC after testing at 900*C in a 1:1:5 ratio methane:oxygen:nitrogen gas mixture. Individual micro SC-SOFC(a), 5 400micron long micro SC-SOFCin series with single interconnect (b), different sized single and multi-interconnect series stack micro SC-SOFC(c), multiinterconnect series stack of micro SC-SOFC(d). 67 To determine if the platinum could have diffused into the YSZ or if it formed some new compound with the YSZ, the samples were placed into an SEM, and EDS was performed to look for the electron scattering signature for platinum within the YSZ. Since the EDS has a penetration depth of several micrometers and the sample's total height is less than 500nm, this technique is able to determine if any platinum is embedded within the YSZ. Figures 34a-b show a close up of a platinum electrode that has become porous. The label in Figure 34a indicates the location of the focused electron energy over the platinum, and Figure 34b shows the matching EDS results. The same process is repeated in one of the pores and is shown in Figures 34c-d. From this data, there appears to be platinum on top of YSZ, however there does not appear to be any within the pore or within the YSZ. Pt YSZ BOe cAe 104dM Cursor Elecron Imse I V,64keV (37 ctmH b a Pt YSZ SIm FIl-c0on c image I ) lS 4 e 1062 cis Crsor 3.104 keV (12 5 cts kIt d Figure 34: SEM and EDS results to investigate platinum electrode. SEM image of porous platinum electrode indicating where EDS measurement is measured on the platinum surface(a), EDS results for measurement over platinum surface confirming the presence of platinum(b), SEM image of porous platinum electrode indicating where EDS measurement is taken within a platinum pore(c), EDS results for measurement within platinum pore, showing no evidence for platinum within the pore(d). 68 Performing EDS over an area where only a small amount of a platinum electrode remains from a multi-interconnect series cell further proves that platinum did not diffuse into the YSZ. The scanned area shows no signs of platinum, Figures 35a-b. These results eliminate the possibility that platinum pores were created by the platinum diffusing into the YSZ. I 5pm Electron Image 1 a 3 2 1 0 u11 Scale 7351 cts Cursor: 2.967 keV (41 cts) 4 5 6 7 8 9 keV b platinum diffusion into YSZ. SEM investigate to results EDS and Figure 35: SEM image of YSZ where platinum electrode had been; box indicates area within which EDS is measured (a), EDS results for measurement YSZ surface, showing no evidence for platinum(b), 69 If the transformation is not formed from platinum diffusion into the YSZ, it is possible that transformation is caused by either a chemical reaction between the electrodes and the gas species, or by an electrochemical reaction facilitated by an electric potential created between the gold and platinum electrodes. Figures 21a-b show the cells after being exposed to nitrogen and oxygen. From these exposures, the gold is essentially unaffected and the platinum has a few small inconsistent defects, which could be influenced by potential dust or other particle contaminants during fabrication. These effects are minimal and do not seem correlated to the formation of the pores in the platinum. Thus, nitrogen or oxygen must not be the sole contributors to the transformation. When the fuel cells are exposed to pure methane, the electrodes transform differently than when exposed to all three gases. Figure 36 is a close up of the micro SC-SOFC exposed to methane after being held at 850*C for over an hour. Under these conditions, the gold electrode transforms, which is not seen during standard operation. The transformation is similar to wetting or melting of gold. When exposed to pure methane, the platinum does not become porous; instead, it acquires small black features. These features could be the result of carbon deposition onto the platinum surface from methane decomposition. XRD results show that gold, platinum, and YSZ seem unaffected but does not indicate the formation of new compounds or carbon deposition, Figure 37. However, this does not discount the possibility for carbon deposition; the carbon could be amorphous, making XRD detection difficult. 70 Figure 36: Micro SC-SOFC exposed to methane at 850C for over one hour. with YSZ N CO N N .D All gas oxygen gas nitrogen gas methane gas 20 30 40 50 60 70 20 (degree) Figure 37: XRD results for samples with gold and platinum on YSZ and exposed to pure methane, pure oxygen, pure nitrogen and a 1:1:5 ratio of methan:oxygen:nitrogen. XRD results only detect platinum, gold and YSZ. 71 The platinum does not appear to diffuse into the YSZ, it does not visibly transform when exposed to nitrogen or oxygen, and methane seems to primarily aid the carbon deposition onto the platinum surface. The last parameter to evaluate is if the transformation occurs due to an electrochemical reaction induced by the gas mixture. To evaluate this, the gold and platinum electrodes were patterned directly onto the silicon substrate. The electrodes were no longer electrochemically coupled by an electrolyte, and thus any transformation cannot be caused by an electrochemical reaction. This experiment was performed twice once with pure nitrogen and again with all three gases, shown in Figures 38a-f and Figures 39a-g, respectively. In both environments, the electrodes are susceptible to transformation; however, the transformation that occurs is different for each environment. In both gas conditions, when the gold and platinum are directly on the silicon, the gold's transformation is very similar to its transformation in the micro SC-SOFCs exposed just to methane as shown in Figure 36. When the platinum on silicon is exposed to the nitrogen at 850*C, only some of the electrodes transformed, whereas when it is exposed to a mixture of gases, all the platinum transforms. However, these transformations are different from those seen with electrodes on an electrolyte. When examining the XRD data for these experiments, Figure 40, it becomes clear that the compound PtSi has formed and can be attributed to the platinum defects observed on silicon, but the data does not indicate that any of the gases influence the deformation, and therefore this does not eliminate the possibility that the pores are caused by an electrochemical reaction. 72 a b d C e f Figure 38: Optical images of Pt-Au patterned directly onto silicon with no electrolyte, exposed to pure nitrogen at various temperatures. Individual micro SC-SOFCs at 400*C no visible changes observed (a), individual micro SC-SOFCs at 700 0 C initiation of platinum transformation (b), series stacks of micro SC-SOFCs at 700C more consistent changes occur to the platinum(c), individual micro SC-SOFCs at 800*C more platinum and all of the gold starts to transform(d), series stacks of micro SC-SOFCs at 800 0 C all platinum and gold transform (e-f). 73 a b c d e f g Figure 39: Optical images of Pt-Au patterned directly onto silicon with no electrolyte, exposed to a 1:1:5 ratio of methane:oxygen:nitrogen at various temperatures. Individual micro SC-SOFCs at 400*C no visible changes observed (a), individual micro SC-SOFCs at 700*C platinum appears to deform (b), series stacks of micro SC-SOFCs at 700*C all platinum in contact with gold does not deform(c), series stacks of micro SC-SOFCs at 750*C platinum in contact and within a certain distance of gold does not transform(d), individual micro SC-SOFCs at 800C more platinum and all of the gold starts to transform(e), series stacks of micro SC-SOFCs at 800*C all platinum and gold transform (f-g). 74 without YSZ n NT 0 en CD C%4 N 0-C)< < CL Nitrogen gas 20 30 40 50 60 70 20 (degree) Figure 40: XRD results for samples with gold and platinum deposited directly on silicon (no YSZ electrolyte) and exposed to pure nitrogen or a 1:1:5 ratio of methan:oxygen:nitrogen. XRD indicates the existence of polycrystalline Pt, Au, and the formation of a new substance PtSi. Regardless of the compounds formed, the changes do not correlate to the transformations documented when the micro SC-SOFCs are exposed to methane, oxygen and nitrogen. This confirms that the platinum transformation is caused by the electrochemical reactions occurring within the fuel cell. Since platinum does not diffuse into the YSZ, and chemical reactions have proven to at most deposit carbon onto the platinum versus removing the platinum from the fuel cell, something is pushing or bubbling through the platinum to create the pores. Revisiting the electrochemical reactions occurring in the fuel cell (Section 4.1), the result of the reaction of methane and oxygen is the formation of water at the anode. Hydrogen has the ability to be absorbed by platinum (53). Therefore when methane decomposes to form carbon dioxide and hydrogen, the hydrogen can be 75 adsorbed by the platinum and travel to the platinum-YSZ interface to meet up with an oxygen anion to form water. At the high operating temperatures, the water is created in vapor form, causing significant expansion and breaks through the platinum electrode. With this understanding, it is important to consider two options. First, if it is desirable to have a solid anode electrode, it would be important to select a stiff material that does not soften at elevated temperatures like platinum. To avoid changing materials to improve the stiffness, having a thicker platinum electrode might alleviate the effects from the water vapor formation. Secondly, if a porous electrode is acceptable or desired, this could resolve the problem, since a porous electrode would be able to accommodate the water vapor formation and allow it to vent without changing the electrodes structure. 4.3 Carbon Nanowire Formation In the data collection process, an interesting phenomenon occurred. Under certain conditions, carbon nanowires were created at the platinum electrode, shown in Figures 41a-c. These were created when exposed to a ratio of 2:1:5 methane:oxygen:nitrogen at 800*C for 15-30mins. Figure 41a shows that every platinum electrode facilitated this carbon nanowire growth. The platinum electrodes on the series stacks degraded away before reaching 800'C, so it is unclear if these electrodes would facilitate the reaction to create carbon nanowires. The entire platinum electrode is involved in the creation of the nanowires. Figure 41b shows that there is no platinum remaining on the surface of the YSZ, while the gold electrode remains unaffected. 76 a b C Figure 41: SEM images of the carbon nanowire formation on the surface of the sample with platinum being the catalysts. At a magnification of 250, it is apparent that platinum electrodes on all individual micro SC-SOFCs have been utilized to form the carbon nanowires (a) at higher magnification of 1000, there is no platinum remaining on the electrolyte, and gold is unaffected (b) at a magnification of 4000, it becomes clear that there is a tangled web of carbon nanowires(c). 77 TEM images show that the platinum is acting as the catalyst and confirms the growth of carbon nanowires versus carbon nanotubes. Figure 42 shows a small platinum particle at the end of the carbon nanowire structure, creating a layering of carbon in a solid configuration. nanotubes. Since there are no center voids, these structures must not be carbon Figure 43 shows that many carbon nanowires can be formed on a large platinum particle. Performing EDS on the nanowires confirms the solid deposition of carbon onto the platinum electrode, Figures 44a-c. Additional investigations will be needed to completely understand the reaction mechanism and determine what can be done to improve the formation of carbon nanowires. Initial speculation is that the new 2:1:5 methane:oxygen:nitrogen gas composition is responsible for the carbon deposition. Figure 45 shows the thermal equilibrium of the chemical species when the chamber has a 2:1 ratio of methane to oxygen. Table 7 provides the approximate mole fractions of the main gas species under this conditions. Table 7: Mole fraction of the primary gas species at thermal equilibrium for a 2:1:5 mixture of methane and oxygen in nitrogen at 800C. H2 CO CH 4 IH 2 0 CO 2 N2 0.61 0.02 0.02 0.05 0.1 Mole Fraction 0.2 78 Figure 42: TEM image of a small platinum particle with a single carbon nanowire growing from its surface. The solid layers of carbon prove the formation of a carbon nanowire verses a carbon nanotube. 79 Figure 43: TEM image of a large platinum nano particle with multiple carbon nanowires growing from its surface. 80 a F 1~4 1C 7 C is is kej b Element Weight% Atomic% CK OK Si K Pt M Au M 515.21 30.37 24.53 74.13 10.09 93.05 4.12 1.89 0.82 0.11 Totals 654.33 C Figure 44: SEM and EDS results to investigate platinum and carbon nanowires. SEM images of gold electrode, YSZ electrolyte and the transformed platinum electrode with carbon nanowires (a), EDS results taken from the platinum and carbon nanowire, showing carbon and platinum (gold is shown because the energy levels for gold and platinum are very similar and the software has trouble distinguishing between the two) (b), depicts the atomic ratio of elements present, indicating substantially more carbon than platinum(c). 81 0.25 020 2 H20 - Equilibrium Mole traction CO2 Equilibrium Mole fraction CO CO- 015- Equilbrium Mole traction H Equilibrium Mole fraction VH4 oiE 0,10 - 005 - -- Equilibrium Mole fraction H2 -9 400 1000 900 800 700 600 500 (C) (C1) Temperature C1 Equilibrium Cluster1 Figure 45: CHEMKIN thermodynamic equilibrium simulation over various temperatures for a 2:1:5 ratio of methane:oxygen:nitrogen The formation of nanowires may be primarily facilitated by a chemical reaction, or by an electrochemical reaction. If a chemical reaction is the basis for this phenomenon, it is most likely the hydrogen reacting with carbon monoxide on the surface of platinum, as shown in Eq. 5. This chemical reaction would deposit solid carbon onto the surface of the platinum. H2 +CO -H 2 0+CS Eq. 5 One study suggests that a high ratio of carbon monoxide to carbon dioxide is responsible for the carbon deposition(54). At equilibrium, methane is present at 800*C when feed gas is 2:1 methane:oxygen, whereas no methane is present when feed gas is in a 1:1 ratio, shown in Figure 29. This suggests that methane could be used to facilitate a chemical or electrochemical reaction to create carbon nanowires. Chemically, Eq. 6 shows how methane could react to form carbon nanowires. CH4 ->C() 82 +2H2 Eq.6 However, methane is probably not the sole cuase of carbon nanowire formation since the micro SC-SOFC operates at 850*C in a pure methane environment and only shows some deposition, Figure 21c, and does not react with every electrode to form carbon nanowires, Figure 42. If formation of carbon nanowires is due to an electrochemical reaction, the anode would have the following half cell reaction, Eq. 7. CH4 +2- -> C() + 2H 20 + 2e- Eq 7 To determine if this reaction controls the formation of the carbon nanowires, a platinum and gold electrode sample patterned directly onto silicon was used to decouple the electrochemical effects. When platinum and gold on silicon were tested with 2:1 methan:oxygen gas mixture at 800*C, very few to no nanowires were formed on the platinum. However, when a SC-SOFC was operating, the fuel cell temperature was higher than the furnace by about 100'C (3, 15). Thus to mimic the increase in operating temperature, the electrodes on bare silicon were tested again at 900*C with the same gas mixture. At 900*C, the platinum on silicon started to produce structures that resembled nanowires. However, the reaction was not uniform. Increasing the temperature to 1 000*C destroyed the platinum and no visible carbon deposits were observed. It appears in some areas that the platinum may have combined with silicon substrate to form PtSi. This investigation is inconclusive regarding the role of electrochemical reactions in the formation of carbon nanowires. The formation of nanowires can be attributed to either the ratio of carbon monoxide to carbon dioxide or electrochemical reactions. Future studies will further investigate this process in greater detail. 83 4.4 Characterization of Individual micro SC-SOFCs 4.4.1 Current-Voltage Data The power density-current density plots with varying electrode lengths shown in Figures 22 and Figure 23 indicate, as expected, that the larger the fuel cell, the higher the power output. In order to compare how the cells perform with respect to each other, the power and current density are normalized by area. Area is computed using the planar configuration area shown in Figure 8. The normalized power and current density data for the 15micron and 5micron gaps are shown in Figure 46 and Figure 47, respectively. A comparison of the max power densities is provided in Figure 48. Power density (area) @ 900C Electrolyte: YSZ Electrodes: Au & Pt Smicron, XXXmicron length with l5micron gap (20:20:100 ccm] CH4:02:N2 Gas: .. ...* 12 l0omicron trial 100micron trial 100micron trial 200rmicron trial trial 0 200micron trial 400micron trial - 400micron trial + 10 + + W * j a200micron 4 a 2 - .. - -. 0 0 a ^ 100 75 50 Currant Density (uA/cm2) 25 I 2 3 1 2 3 1 2 125 Figure 46: Power density versus current density for 5micron wide electrodes separated by a gap distance of 15microns, experimented at 900C in a 1:1:5 methane:oxygen:nitrogen environment. Power Density (area)@ 900C Electrolyte: YSZ Electrodes: Au & Pt 5micron, XXXmicron length with Smicron gap Gas: CH4:02:N2 [20:20:100 ccm) 20 18a - 1 6 14 P 4 g2r 100emcaon trialed d aa gam a b r mtaellntcrrgn Curntdntt (Acm2 0 0 25 s0 7S craptrial m2 100 120 IS0 175 200 225 250 Carrat donsity taA/cma2) Figure 47: Power density versus current density for 5micron wide electrodes separated by a gap distance of 5microns, experimented at 900'C in a 1:1:5 methane: oxygen: nitrogen environment. 84 SC-SOFCs with electrodes separated by 5 microns show higher power density than those with electrodes separated by 15 microns. This is expected since gap area increases overall fuel cell area but is catalytically inactive and does not contribute to power. Additionally, increasing the gap area increases the electrolyte resistance. The normalization also shows that for each gap distance, the max power density falls within the same value range when accounting for experimental uncertainty, Figure 48. Maximum Power Density versus Fuel Cell Area 20 16 1E14 12 10- 1.E 05 2,E-C5 3.5-CS 4.E-05 55 E05 6 E-05 7.E-05 8.505 9.E 35 :.E-34 Fuel Cell area (cm^2) S13micron Gup * 5micron~ Gup Figure 48: Power density vs. fuel cell area. For each electrode gap distance, there is a given power density that is within the same range for the different fuel cell sizes. There are some clear benefits for moving to a through-plane geometry. As discussed in Section 1.4.3, the "active area" for planar SC-SOFCs is defined to be the area of both electrodes and the area that separates the electrode. A micro SC-SOFC in the planar configuration with 5 micron wide electrodes separated by a 5 micron gap exhibits a final power density at least 3 times lower than that of the analogous through-plane configuration. In addition, electrolyte resistance decreases by several orders of magnitude in a through-plane SC-SOFC, as the length decreases and the cross-sectional area increases. Resistance reduction improves fuel cell performance. Although the above analysis is overly simplified, it is clear that there are potential benefits for moving to a 85 through-plane geometry. However, such geometry requires more complex fabrication methods and may have other complications such as higher mass flow limitations. Assuming Hibino, et. al.(19) correctly calculated the area for both the planar and throughplane SC-SOFC configurations, Figure 49 (Figure 9 and Figure 12) shows that going from a planar to through-plane SC-SOFC design may not increase the power output. Assuming that the electrolytes conductivity, cross-sectional area, and path lengths are the same for the planar and through-plane configuration, the power output does not increase by a factor of three. This suggests that a through-plane configuration has additional limitations. However the performance of a through-plane SC-SOFC can be improved by reducing the thickness of the electrolyte layer. 800 0( E MY) 0 40 -f fE f I) b a 12 showing IV data for Figure Figure 49: A side by side comparison of Figure 9 and planar (a) and through-plane(b) SC-SOFCs( 19) 4.4.2 AC Impedance Data As seen in Section 3.4, the data show a consistent electrolyte resistance for each fuel cell under each testing condition. The previous observation made in Section 4.4.1 about the reduction in power density with respect to an increase in the gap between electrodes is validated using AC impedance by measuring the electrolyte resistances. Using the data from Figures 27a-b, a resistance ratio between the fuel cells with a 5, 10, and 15Smicron electrode gap distance is created to indicate how these resistances scale with gap size. The ratios for the average resistance and electrolyte resistance are shown in Figure 50. Both the average resistance and electrolyte resistance increase almost linearly with the electrode gap distance. 86 This expected trend validates the current-voltage data results predicted in Section 1.4.4 and shown in Table 3. The trend between electrolyte resistance and fuel cell size is also shown experimentally, Figure 26b. Even though the predicted trends are validated experimentally, the measured value for the electrolyte resistance is an order of magnitude larger than that calculated. This suggests that either the selected YSZ conductivity does not match that of the experimental YSZ, experimental tolerance for measuring within the high frequency domain (the domain that provides information about the electrolyte) is a factor, or the conductivity is suffering from the electrolyte being too thin to allow the anions to travel freely through the electrolyte as demonstrated in Figures 10-11 and as proposed by Chung (13). If the electrolyte is indeed too thin to function as predicted in Eq. 3, a new thin film conductance model would be needed to adequately predict the electrolyte resistance for planar micro-SC-SOFC devices. Normalized Change in Resistance as Separation of Electrodes Increases 3.50 3.00 2.0 2.50 M 2.00 U.2 2 L VS 1.50 1.00 0.50 0 0.00 5 micron 10 micron 15 micron Electrode Separation Distance (microns) - --*- Electrolyte Resistance Ratio Electrode Resistance Ratio ideal Resistance Ratio Figure 50: resistance ratios with respect to electrode gap separation. To determine the rate limiting electrode, an 'Area Specific Resistance'(ASR) is extracted from the AC impedance data. A control experiment was performed using a micro SCSOFC with 15 micron wide electrodes. When one electrode is changed to 5 microns, the change in resistance can be attributed to the change in area of that specific electrode. Multiplying the change in resistance by the change in area can provide an ASR for that electrode. Applying this technique to the data shown in Figure 25a provides the following ASR for the platinum and gold electrodes, Table 8. 87 Table 8: Computed values for the change in resistance and the ASR attributed to specific electrodes. Platinum (5_15) Gold (15_5) Change in resistance (AQ) [(15-15) - (xx-yy)] Electrode specific ASR 1.03E+07 Q 3.87E+08 Q 412 Qcm 2 15463 Qcm 2 (AQ*AArea) The platinum and gold ASR values are both several orders of magnitude larger than what has been reported in literature(55). One potential reason for the discrepancy is the size difference between electrodes. Herle, et. al. used gold electrodes that were 20 microns thick with a cross-sectional area of 2.27cm 2 . In comparision, the micro SC-SOFCs have thin film electrodes that are 200 nanometers thick, occupying an area several orders of magnitude smaller. Since the gold is a dense thin film, the reduction in surface area from a larger electrode can reduce its reactivity. Additionally this can be indicating the poor selectivity of gold and platinum catalysts. Since the platinum electrode is porous, it can reduce the resistance; however, platinum is susceptible to coking which can reduce the reaction sites, resulting in higher resistance. If the gold were porous, it could be more reactive and take on properties similar to gold nanoparticles, which might reduce its resistance(52). With the ASRs from Table 8, an average electrolyte resistance obtained from Figure 25b, and assuming an OCV of 0.4V, a current-voltage plot can be generated to show the effect of the resistances on fuel cell performance, shown in Figure 51. In the case where there are no losses, the voltage would remain constant over all current ranges. However, with the introduction of the electrolyte resistance, performance does decrease slightly, but the effect is often negligible. The same can be said with respect to the losses attributed to the platinum electrode. The gold cathode, however, creates the largest resistance and limits the current output. This makes sense for several reasons: firstly, the gold electrode is not porous so it has less surface area available for reaction. Secondly, oxygen reduction is known to be a slow and rate limiting process. Lastly, gold can reduce oxygen, but is ineffective. Materials such as BSCF and LSF are much better at reducing oxygen than gold and will be investigated in future studies (34). When comparing the theoretical current-voltage data shown in Figure 51 to collected data in Figure 52, a close 88 correlation can be seen between the analytical model and experimental data. This validates the approach to extracting an ASR value as well as the methods used to characterize fuel cell operation and relate the information directly to fuel cell performance. Finally, to emphasize how much improvement is needed at the cathode, Table 9 provides the ratios of resistances used to create Figure 51. Table 9 shows that the cathode resistance is tens to hundreds of times larger than the electrolyte or platinum resistances. Current-Voltage Predictions from Experimentally Determined Electrode ASR: Platinum/Gold Electrodes 5micron by 400micron & Platinum/Gold Electrodes 15micron by 400micron 0.45 0.4;,- 0.35 y 0 IM no v loss A 0.3 . 25 PtAu Aa . A S0.2 Electrolyte Pt&Au 15micron, Cathode Losses AA > 0.15 A 0.1 I5micron, Losses Pt&Au iSmicron, Anode Losses A A 0 Pt&Aul Smicron, electrolyte Losses Pt&Au Smicron, Anode Losses A Pt&Au Smicron. Cathode Losses A 0.05 0 0.OOE+00 2,OOE-10 4,00E-10 6.OOE-10 8.OOE-10 Current (A) 1.OOE-09 1.20E-09 1.40E-09 Figure 51: Analytical model of a micro SC-SOFC with 5micron and 15micron wide gold and platinum electrodes. The parameters for this model are based on extracted ASR values for the platinum and gold electrodes at 700*C. Voltage verse Current for 15micron by 400micron micro SC-SOFC at 700C in 1:1:5 CH4:02:N2 0.410.3 i0.2 0 0.00E+00 1.OOE-10 2.00E-10 3.00E-10 4.00E-10 5.00E-10 6.00E-10 7.OOE-10 8.00E-10 9.00E-10 1.00E-09 Current (A) Figure 52: Experimental data for a micro SC-SOFC with 15micron wide gold and platinum electrodes at 700 0 C in a 1:1:5 ratio of methane:oxygen:nitrogen. 89 Table 9: Comparison of electrode and electrolyte resistances. Pt Q/ YSZ Q Au Q /Pt Q Au Q /YSZ i Pt Au 15micron_1 5micron 37.51 193.12 5.15 5micron_5 micron 37.51 579.37 15.45 90 4.5 Stacked SC-SOFC scaling One of the ultimate goals of this project is to be able to obtain high voltage stacks in a very small area. Figure 28 shows the initial data evaluating a stack of 10 micro SCSOFCs attached in series. The OCV across different quantities of micro SC-SOFCs stacked in series is shown in Figure 53, and can be fit to a trend line with slope of 0.33. This agrees with conventional theory and it behaves similarly to reported trends in literature(21, 26, 37). The average voltage per number of cells in the stack is approximately 0.325V for stack sizes of 4 or more, Figure 54. This average voltage is similar to the single cell tests previously mentioned as well as the voltages reported in literature. To my knowledge, this is one of the highest numbers of fuel cells in a SCSOFC stack to date that maintains a consistent voltage across all of its cells. The slight reduction in voltage for the individual and 2 cells stacked in series may possibly be attributed to cell degradation since they were the last cells to be tested. The degradation of stacked cells is a problem that will need to be addressed in futures studies. Stack Voltage for 'n' Cells 3.50 - 3.00 y = 0.3304x R 2.50 2 0.985 > 2.00 U 0 . 150 1.00 0.50 0.00 0 2 4 6 8 10 Number of Cells in a Stack Figure 53: OCV across different quantities of micro SC-SOFCs stacked in series. 91 Unit Cell OCV for "n" Stacked Cells 0.4 U 0.35 r 0.3 U=0.25 0ow0U n4-0.2 M 0 - 0.1 U (U 4'0.05 0 0 2 4 6 8 10 12 # of Cells in a stack Figure 54: average OCV across different quantities of micro SC-SOFCs stacked in series. 92 5.0 Summary This work demonstrates several novel and insightful results of individual and stacked micro SC-SOFCs using noble metal electrodes: First, a change in direction of the OCV at 600'C has indicated a change in the chemical species exposed to the fuel cell electrodes. This suggests that different electrochemical reactions are occurring on the gold and platinum depending on temperature and gas composition. At low temperatures, the gold and platinum electrodes are respectively performing the anodic (CO+0 2 -> CO 2 + 2e~) and cathodic (/202 + 2e- -> 02.) reactions, while at high temperatures the gold and platinum are hypothesized to be respectively performing the cathodic (V20 and anodic (H2+02- -> 2+ 2e~ -> 02-) H20 + 2e-) reactions. To my knowledge, this is the first reported electrochemical potential reversal as a function of temperature. These findings suggest the importance of electrode materials and gas composition. Second, higher temperature results in better performance. For an individual micro SCSOFC at 900'C, an OCV of ~0.4V and a peak power density of 27p,W/cm2 was achieved. There is a lot of room for improvement. Experiments indicate that having the smallest electrode-electrode separation is desirable. The cathodic reaction is shown to be rate limiting; therefore, increasing the size of the cathode by several factors may be able to make up for its inadequate performance. Third, at 800'C, using a 2:1 ratio of methane to oxygen, the platinum electrode has shown coking to form carbon nanowires. It is unclear at this time if this finding is novel, or has any new applications. However, this is a distinct byproduct of this study and is worth investigating in the future. Lastly, a stack of 10 micro SC-SOFCs in series produces an OCV of 3.3V, where each fuel cell has an average OCV of approximately 0.33V. This validates the micro fabrication methods utilized to create micro SC-SOFC stacks, and has provided a method to easily create the largest SC-SOFC stack reported. This proof of concept will be extended in the future to further increase the number of cells in a stack. 93 Among these experimental results, it is important to consider the potential of the micro fabrication process developed to create individual and stacks of micro SC-SOFCs. By utilizing the micro-fabrication techniques of sputtering and photolithography outlined in Section 2.3, it will be possible to make hundreds or thousands of stacked fuel cells in an area of less than one square centimeter. This work validates the micro fabrication stack concept. Comparing the collected data to literature, it becomes apparent that our lack of performance can be largely attributed to the poor selectivity of the gold and platinum electrodes. Therefore, with the use of more selective materials and an optimized geometry design, this technology will gain potential to become commercially viable. Future studies will go in two directions: materials and design. The micro SC-SOFCs will be fabricated using materials with enhanced selectivity such as BSFC and SDC. This should increase fuel cell performance by several orders of magnitude, from 1x0~5 watts per square centimeter to lx10.1 watts per square centimeter. Using these new materials will also reduce the operating temperature of micro SC-SOFCs. In addition, future work will further develop planar design and different stacking structures, such as the throughplane configuration shown in Figure 7, as well as other geometric layouts including three-dimensional structures and contoured surfaces. These innovations have the potential to create a system that is not only simple, inexpensive, and robust, but also one that has adequate power density. If micro SC-SOFCs can be improved to have a comparable power density to a traditional SOFC, then micro SC-SOFCs may be able to break down the current barriers to entry of fuel cell technology into commercial applications. 94 95 96 References 1. Linden, D.R., Thomas B., Handbook of Batteries. Third Edition ed, ed. D.R. Linden, Thomas B. 2002: McGraw-Hill. 2. Burke, K.A., Fuel Cells for Space Science Applications. American Institute of Aeronautics and Astronautics, 2003. 2003-5938. 3. Hibino, T., A. Hashimoto, T. Inoue, J. Tokuno, S. Yoshida, and M. Sano, A solid oxide fuel cell using an exothermic reaction as the heat source. Journal of the Electrochemical Society, 2001. 148(6): p. A544-A549. 4. Shao, Z.P. and S.M. Haile, A high-performance cathode for the next generation of solid-oxidefuel cells. Nature, 2004. 431(7005): p. 170173. 5. Larminie, J. and A. Dicks, Fuel Cell Systems Explained 2nd Edition. book, 2003. 6. Allen J. Bard, L.R.F., Electrochemical Methods Fundamentals and Applications. Vol. 2nd edition. 2001, Hoboken, NJ: John Wiley & Sons, Inc. 7. Yan, J., H. Matsumoto, M. Enoki, and T. Ishihara, High-Power SOFC Using La[sub 0.9]Sr[sub 0.1]Ga[sub 0.8]Mg[sub 0.2]0[sub 3 delta]/Ce[sub 0.8]Sm[sub 0.2]0[sub 2 - delta] Composite Film. Electrochemical and Solid-State Letters, 2005. 8(8): p. A389-A391. 8. Ahn, S.-J., J. Moon, J.-H. Lee, and J. Kim, Single-chambersolid oxide micro-patterned interdigitated electrodes. cell with fuel Electrochemical and Solid State Letters, 2006. 9(5): p. A228-A23 1. 9. Asahara, S., D. Michiba, M. Hibino, and T. Yao, Single Chamber SOFC Using BaLaIn[sub 2]O[sub 5.5] Solid Electrolyte. Electrochemical and Solid-State Letters, 2005. 8(9): p. A449-A45 1. 10. Buergler, B.E., M.E. Siegrist, and L.J. Gauckler. Single chamber solid oxide fuel cells with mixed ionic electronic conducting electrolyte. 2004. Honolulu, HI, United States: Electrochemical Society Inc., Pennington, NJ 08534-2896, United States. 11. Buergler, B.E., M.E. Siegrist, and L.J. Gauckler, Single chamber solid oxide fuel cells with integrated current-collectors. Solid State Ionics, 2005. 176(19-22): p. 1717-1722. 97 12. Choi, S.H., W.S. Kim, H.Y. Jung, S.-J. Ahn, J.-H. Lee, H.-W. Lee, and J. Kim. Structure and conductingproperties of porous NiO films for single chamber micro-solid oxide fuel cell. 2005. Quebec, Canada: Electrochemical Society Inc., Pennington, NJ 08534-2896, United States. 13. Chung, C.Y. and Y.C. Chung, Performance characteristicsof micro single-chamber solid oxide fuel cell. Computationalanalysis. Journal of Power Sources, 2006. 154(1): p. 35-41. 14. Hao, Y., C. Pantano, and D.G. Goodwin. A two-dimensional model of a single-chamber SOFC with hydrocarbon fuels. 2005. Quebec, Canada: Electrochemical Society Inc., Pennington, NJ 08534-2896, United States. 15. Hao, Y., Z. Shao, J. Mederos, W. Lai, D.G. Goodwin, and S.M. Haile, Recent advances in single-chamber fuel-cells: Experiment and modeling. Solid State Ionics, 2006. 177(19-25): p. 2013-2021. 16. Hibino, T., Hashimoto, A., Yano, M., Suzuki, M., Yoshida, S., Sano, M., High Performance Anodes for SOFCs Operating in Methan-Air Mixture at Reduced Temperatures. Journal of Electrochemical Society, 2002. 149(2): p. A133-!136. 17. Hibino, T., A. Hashimoto, T. Inoue, J. Tokuno, S. Yoshida, and M. Sano, Single-chamber solid oxide fuel cells at intermediate temperatures with various hydrocarbon-airmixtures. Journal of the Electrochemical Society, 2000. 147(8): p. 2888-2892. 18. Hibino, T., A. Hashimoto, T. Inoue, J. Tokuno, S. Yoshida, and M. Sano, A low-operating-temperature solid oxide fuel cell in hydrocarbon-airmixtures. Science, 2000. 288(5473): p. 2031-2033. 19. Hibino, T., H. Tsunekawa, S. Tanimoto, and M. Sano, Improvement of a single-chamber solid-oxide fuel cell and evaluation of new cell designs. Journal of the Electrochemical Society, 2000. 147(4): p. 1338-1343. 20. Hibino, T., K. Ushiki, and Y. Kuwahara, New conceptfor simplifying SOFC system. Solid State Ionics, 1996. 91(1-2): p. 69-74. 21. Hibino, T., K. Ushiki, T. Sato, and Y. Kuwahara, A Novel Cell Design for Simplifying Sofc System. Solid State Ionics, 1995. 81(1-2): p. 1-3. 22. Hibino, T., S.Q. Wang, S. Kakimoto, and M. Sano, Single chamber solid oxide fuel cell constructed from an yttria-stabilized zirconia 98 electrolyte. Electrochemical and Solid State Letters, 1999. 2(7): p. 317-319. 23. Hibino, T., S.Q. Wang, S. Kakimoto, and M. Sano, One-chamber solid oxide fuel cell constructed from a YSZ electrolyte with a Ni anode and LSM cathode. Solid State Ionics, 2000. 127(1-2): p. 89-98. 24. Hori, M., K. Nagasaka, M. Miyayama, G. Trunfio, and E. Traversa, Evaluation of electrode performances of single-chamber solid oxide fuel cells, in Electroceramics in Japan Viii. 2005. p. 155-158. 25. Hori, M., K. Nagasaka, M. Miyayama, G. Trunflo, and E. Traversa, Evaluation of electrode performances of single-chamber solid oxide fuel cells. Key Engineering Materials, 2006. 301: p. 155-158. 26. Horiuchi, M., S. Suganuma, and M. Watanabe, Electrochemical power generation directly from combustion flame of gases, liquids, and solids. Journal of the Electrochemical Society, 2004. 151(9): p. A1402-A1405. 27. Hyoungchul, K., S.H. Choi, W.S. Kim, J.-H. Lee, H.-W. Lee, and J. Kim. Development of single chamber solid oxide fuel cells with coplanar micro-electrode array. in 207th Meeting of the Electrochemical Society. 2005. Quebec, Canada: Electrochemical Society. 28. Jacques-Bedard, X., T. W. Napporn, R. Roberge, M. Meunier, Coplanar Electrodes Designfor a Single-Chamber SOFC. Journal of Electrochemical Society, 2007. 154(3): p. B305-B309. 29. Jacques-Bedard, X., T.W. Napporn, R. Roberge, and M. Meunier, Performance and ageing of an anode-supportedSOFC operated in single-chamber conditions. Journal of Power Sources, 2006. 153(1): p. 108-113. 30. Jasinski, P., T. Suzuki, F. Dogan, and H.U. Anderson, Impedance spectroscopy of single chamber SOFC. Solid State Ionics, 2004. 175(1-4): p. 35-38. 31. Napporn, T.W., X. Jacques-Bedard, F. Morin, and M. Meunier, Operating conditions of a single-chamber SOFC. Journal of the Electrochemical Society, 2004. 151(12): p. A2088-A2094. 32. Napporn, T.W., F. Morin, and M. Meunier, Evaluation of the actual working temperature of a single-chamber SOFC. Electrochemical and Solid State Letters, 2004. 7(3): p. A60-A62. 99 33. Napporn, T.W., S. Savoie, R. Roberge, X. Jacques-Bedard, and M. Meunier. Single-chamber SOFC. Comparing dry and humidified conditions. 2005. Quebec, Canada: Electrochemical Society Inc., Pennington, NJ 08534-2896, United States. 34. Shao, Z., J. Mederos, W.C. Chueh, and S.M. Haile, High powerdensity single-chamber fuel cells operated on methane. Journal of Power Sources, 2006. 162(1): p. 589-596. 35. Shiratori, Y. and Y. Yamazaki, Study of high voltage single chamber SOFC with series connected cells I. IV-IF characteristics of two segments cell. Electrochemistry, 2001. 69(2): p. 92-97. 36. Stefan, I.C., C.P. Jacobson, S.J. Visco, and L.C. De Jonghe, Single chamber fuel cells: Flow geometry, rate, and composition considerations.Electrochemical and Solid State Letters, 2004. 7(7): p. A198-A200. 37. Suzuki, T., P. Jasinski, H.U. Anderson, and F. Dogan, Single chamber electrolyte supported SOFC module. Electrochemical and Solid State Letters, 2004. 7(11): p. A391-A393. 38. Suzuki, T., P. Jasinski, H.U. Anderson, and F. Dogan, Role of composite cathodes in single chamber SOFC. Journal of the Electrochemical Society, 2004. 151(10): p. A1678-A1682. 39. Suzuki, T., P. Jasinski, V. Petrovsky, H.U. Anderson, and F. Dogan, Anode supported single chamber solid oxide fuel cell in CH4-air mixture. Journal of the Electrochemical Society, 2004. 151(9): p. A1473-A1476. 40. Suzuki, T., P. Jasinski, V. Petrovsky, H.U. Anderson, and F. Dogan, Performance of a porous electrolyte in single-chamber SOFCs. Journal of the Electrochemical Society, 2005. 152(3): p. A527-A531. 41. Tomita, A., S. Teranishi, M. Nagao, T. Hibino, and M. Sano, Comparative performance of anode-supported SOFCs using a thin CeO. 9GdO. 101.95 electrolyte with an incorporatedBaCeO.8Y0. 203alpha layer in hydrogen and methane. Journal of the Electrochemical Society, 2006. 153(6): p. A956-A960. 42. Ahn, S.-J., Kim Yong-Bum., Moon, Jooho., Lee, Jong-Ho., Kim, Joosun, Co-planar type single chamber solid oxide fuel cell with micro-patterneed electrodes. Journal of Electroceram, 2006. 17: p. 689-693. 100 43. Yamamoto, 0., Solid State Electrochemistry, Chapter II "Applications",ed. P.G. Bruce. 1995: Cambridge University Press. 44. Ahn, S.-J., J. Moon, J.-H. Lee, and J. Kim. Single-chamber solid oxide fuel cell with micro-patternedinterdigitatedelectrodes. 2005. Quebec, Canada: Electrochemical Society Inc., Pennington, NJ 08534-2896, United States. 45. Hibino, T., K. Asano, and H. Iwahara, Solid Oxide Fuel-Cell Which Can Work in Uniform-Gas Phase Using Partial Oxidation of Methane. Nippon Kagaku Kaishi, 1994(7): p. 600-604. 46. Macdonald, J.R., Impedance Spectroscopy Emphasizing Solid Materials and Systems, ed. J.R. Macdonald. 1987, New York: John Wiley & Sons. 47. Buergler, B.E., A.N. Grundy, and L.J. Gauckler, Thermodynamic equilibrium of single-chamber SOFC relevant methane-air mixtures. Journal of the Electrochemical Society, 2006. 153(7): p. A1378A1385. 48. Virkar, A.V., J. Chen, C.W. Tanner, and J.W. Kim, The role of electrode microstructure on activation and concentration polarizations in solid oxide fuel cells. Solid State Ionics, 2000. 131(12): p. 189-198. 49. Cheng, X., Z. Shi, N. Glass, L. Zhang, J. Zhang, D. Song, Z.-S. Liu, H. Wang, and J. Shen, A review of PEM hydrogen fuel cell contamination: Impacts, mechanisms, and mitigation. Journal of Power Sources, 2007. 165(2): p. 739-756. 50. Berlowitz, P.J., C.H.F. Peden, and D.W. Goodman, Kinetics of carbon monoxide oxidation on single-crystal palladium, platinum, and iridium. J. Phys. Chem., 1988. 92(18): p. 5213-522 1. 51. Grisel Rudd, K.-J.W., Andrea Gluhoi, Bernard E. Nieuwenhuys, Catalysis by Gold Nanoparticles. Gold Bulletin, 2002. 35(2): p. 3945. 52. Haruta, M., Size- and support-dependency in the catalysis of gold. Catalysis Today, 1997. 36(1): p. 153-166. 53. Markovic, N.M., Ross, P.N. Jr., , Surface science studies of modelfuel cell electrocatalysis.Surface Science Reports, 2002(45): p. 117-229. Murray, E.P., T. Tsai, and S.A. Barnett, A direct-methanefuel cell with a ceria-basedanode. Nature, 1999. 400(6745): p. 649-651. 54. 101 55. 102 Huang Keqin, P.Y.H., John B. Goodenough, Characterizationof ironbased alloy interconnects for reduced temperature solid oxid fuel cells. Solid State Ionics, 2000. 129: p. 237-250. Appendix A. Temperature Dependence on Ion Conductivity for Selective Oxides Temperature () 800 1000 900 1200 s7-4T 100 700 j 600 *ZI, E 102 S J/L7~ C C 10 60 8.0 10.0 12.0 10IT(K ') Temperature Dependence on Ion Conductivity for Selective Oxides (43) 103