CHEM A112 – General Chemistry II – Spring 2016
advertisement

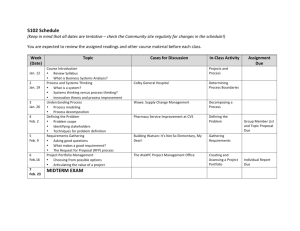
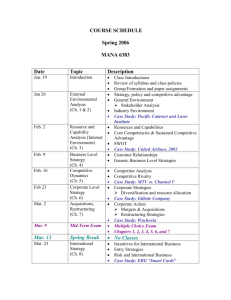
CHEM A112 – General Chemistry II – Spring 2016 M,W,F – 12n - 1:50p SBDG 301 Dr. Kenneth Roberts SBDG 322A; ph: 803/641-3561; e: kenr@usca.edu Office Hours: M-F – 9a - 10a or by Appointment CHEM A112 – General Chemistry II (4) (Prereq: CHEM 111 or consent of the department) The second portion of a two-semester sequence of courses that introduces the principles, vocabulary and methods of chemistry appropriate for those in engineering, mathematical and natural sciences. This course finishes the first year of General College Chemistry. ATTENDANCE: University and departmental guidelines apply. A student cannot miss more than 25% of all lectures and laboratories to pass the course. If a student has more than two (2) unexcused absences, a penalty of the loss of one letter grade will result. Roll will be taken through quizzes and lab flow charts along with an attendance sheet. Excused absences must be verified with a doctor’s note, a note from a family member that includes a telephone number, or a business note. Arrangements will be made to deal with the material missed for excused absences only. Regardless of reason, the student is responsible for the missing course and laboratory content. TEXTBOOKS: “Chemistry”, 2015 Ed. by OpenStax College, Rice University, Houston Texas, Content Leaders: Flowers, Theopold & Langley “General Chemistry Laboratory Manual” by USCA Chemistry Faculty, 2015 (Lavender Cover) “Study Manual for ACS Standardized Tests,” by the American Chemical Society ALSO REQUIRED: Scientific Calculator - for class as well as homework and exams! Graphing capabilities are not necessary Closed-toe shoes and long pants for laboratory - every lab day; safety goggles will be supplied Laboratory notebook with carbonless paper Routine access to BlackBoard – problem sets, answer keys, syllabus, grade scores, course announcements, etc. COURSE OBJECTIVES The introduction of topics such as: 1) general chemical equilibrium; 2) acid/base, complex formation, and solubility equilibria; 3) kinetics; 4) free energy; 5) electrochemistry; 6) nuclear chemistry; and 7) organic and biochemistry are planned. The three main characteristics of chemistry will be stressed in this course: (1) chemical nomenclature (vocabulary and formulae); (2) chemical concepts (e.g. reaction kinetics, thermodynamics, acid/base chemistry, and equilibria); and (3) chemical methods (including mathematical manipulations, procedures, and complex problems, as well as laboratory techniques). This course is intended to provide the topics, concepts and skills that lay the foundations for chemistry and to introduce the ways in which chemistry overlaps with other scientific disciplines. Additionally, it is my intent to imbue the student with confidence in the routine use of chemistry concepts and calculations; igniting a curiosity and excitement for chemistry is a personal bonus. My intention is that lectures will follow the book closely with a tentative course schedule attached. Practical laboratory exercises will be integrated within the lecture period, and are intended to apply directly to the material being covered. COURSE EVALUATION There will be ten smaller tests given throughout the semester. Please see me if a test is missed due to absence. An absence must fit the criteria for an excused absence (see above under ATTENDANCE) for a makeup exam to take place. The tentative dates for tests are provided in the attached course schedule. Pre-lab (completed before each scheduled lab) and lab reports (turned in at the beginning of next class) will be used to determine the lab portion of your grade. The pre-lab will be done in your notebook PRIOR to coming to class and will be checked before leaving the classroom. Specific details for the laboratory will be given in class. The lab report requirements are given at the end of each lab manual experiment/procedure, with the addition that a 1-2 paragraph summary conclusion be included at the end. The Safety Rules for each lab will be reviewed. Any repeated safety infraction during lab may result in a dismissal for that day’s work. Approved eye protection must be worn at all times in the lab. A Final Exam will be given in our regular classroom (SBDG 301) on Friday, April 29 at 2p. The Final Exam is the American Chemical Society Exam for General Chemistry. This national exam covers CHEM A111 and A112, is timed, and is comprehensive. We will use the required Study Guide throughout the semester in preparation for this exam. Electronic devices including, but not limited to phones, tablets, laptops, etc. will NOT be permitted in the classroom during exams. Anyone caught with or using such device(s) will be given a grade of 0 for that exam. GRADING FORMAT AND POLICIES: Lecture: Mini-Tests (10 @ 50 pts) Final Exam (100 pts) 500 pts 100 pts Laboratory: Demos Pre-Labs Lab Reports Total: (4 @ 4 pts) (16 @ 3 pts) (16 @ 6 pts) 16 pts 48 pts 96 pts 760 pts Final grades: Based on the percentage of points out of a total of 748; A ≥ 90% B ≥ 80% C ≥ 68% D ≥ 60% The ACADEMIC HONOR CODE is in place for all assignments and exams. The Honor Code states that it is dishonest to either give or receive any unauthorized aid or assistance on an exam or homework. Evidence of such practice is very obvious to a grader, so be forewarned; such behavior will result in a zero for the assignment or exam. A student must complete the work in the laboratory and take the final to pass this class. Thursday, March 31, 2016 is the last day to drop this class without receiving a WF for the course. Note: Any student who has a physical, psychological, and/or learning disability that might affect your performance in this class or lab, please contact the Office of Disability Services as soon as possible. If you are registered with that office and have an accommodations sheet, please see me after class today or phone/email me as soon as possible to set up an appointment. CHEM A112 – General Chemistry II – Spring 2016 M,W,F – 12n - 1:50p SBDG 301 Date Lecture Dr. Kenneth Roberts SBDG 322A, x3561 E-mail: kenr@usca.edu Office Hours: M-F – 9a - 10a or by Appointment Laboratory Date Lecture Jan 11 M Syllabus; Chapter 19 Mar 2 W Chapter 15 Jan 13 W Chapter 19 Mar 4 F Chapter 15 Jan 15 F Chapter 19 Jan 18 M MLK DAY – NO CLASS Beer’s Law (pg. 88) Mar 14 M Chapter 15 Mar 16 W Chapter 15; Test #6 Mar 18 F Chapter 15 Selective Precipitation (pg. 125) Coupled Equilibria (pg. 127) Chapter 12 Jan 22 F Chapter 12; Test #1 Jan 25 M Chapter 12 DEMO: Iodine Clock (pg. 98) Mar 21 M Chapter 15 Jan 27 W Chapter 12 Candle Height Kinetics (Handout) Mar 23 W Chapter 16 Rate Law: Crystal Violet (pg. 94) Jan 29 F Chapter 12 Chapter 13; Test #2 Feb 3 W Chapter 13 Feb 5 F Chapter 13 DEMO: Le Chatelier’s (pg. 104) Mar 25 F Chapter 16; Test #7 Mar 28 M Chapter 16 Entropy (pg. 129) Mar 30 W Chapter 16 Last Drop Date (Mar 31) Apr 1 F Chapter 17 Feb 8 M Chapter 13 Apr 4 M Chapter 17; Test #8 Feb 10 W Chapter 13; Test #3 Apr 6 W Chapter 17 Feb 12 F Chapter 14 Apr 8 F Chapter 17 Feb 15 M Chapter 14 Apr 11 M Chapter 17 Feb 17 W Chapter 14 pH: Items; Acids/Bases (pg. 110; 111) Apr 13 W Chapter 17; Test #9 Acidic/Basic Salts (pg. 114) Feb 19 F Chapter 14; Test #4 Feb 22 M Chapter 14 Feb 24 W Chapter 14 Feb 26 F Chapter 14 Feb 29 M Chapter 14; Test #5 Finding Kc (pg. 106) Mg(OH)2 Solubility (pg. 123) SPRING BREAK - NO CLASS Jan 20 W Feb 1 M Spectrochemical Series (pg. 92) Mar 7-11 Laboratory Apr 15 F Chapter 21 Apr 18 M Chapter 21 Buffers (pg. 116) Apr 20 W Chapter 20 DEMO: Titration/Wk Acid (pg. 119) Apr 22 F Chapter 20; Test #10 Apr 25 M Chapter 20 Redox Titration (Handout) Redox Potentials (pg. 131) Radioisotope Decay (pg. 135) DEMO: Wintergreen (pg. 137) American Chemical Society General Chemistry Final Exam – 2PM, Friday, April 29, 2016