Science SCI.V.4.3 Grade: 9
advertisement

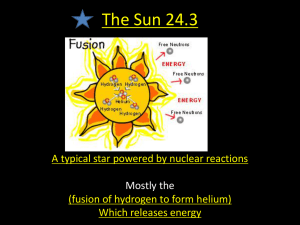
Science Grade: 9th SCI.V.4.3 Strand V: Using Scientific Knowledge in Earth Science Standard 4: Solar System, Galaxy and Universe- All students will explain scientific theories as to the origin of the solar system Benchmark 3: Explain how stars and planetary systems form and how stars produce energy. Constructing and Reflecting: SCI.I.1.2 – Design and construct scientific investigations. SCI.I.1.4 – Gather and synthesize information from books and other sources of information. SCI.I.1.5 – Discuss topics in groups by making clear presentations, restating or summarizing what others have said, asking for clarification or elaboration, taking alternative perspectives, and defending a position. SCI.II.1.1 – Justify plans or explanations on a theoretical or empirical basis. Vocabulary Context • • • • • • • • • • • • • • Hydrogen Helium Coalescence Nebula Nova Fusion Radiation Fission • Nebulas considered to be star forming regions Supernovas Nuclear fusion research Heavy and light elements Hot interiors of the earth-like planets Age of the solar system ¾ Explosions of stars producing heavy elements o Hydrogen o Helium Process of formation ¾ Coalescence from clouds of dust and gases by gravity. Knowledge and Skills Students will: • Explain how the gravitational collapse of a cloud of gas and dust produces extreme pressure and temperature that triggers nuclear fusion • Explain how smaller atoms combine to make larger ones during nuclear fusion, release large quantities of energy, and form a star • Explain how heavy elements have been spread throughout the universe Resources Coloma Resources: Glencoe CH 29 Our Solar System Glencoe MiniLabs Pg 116 Scaling The Solar Sys. Glencoe Labs 29.1 Your Age & Weights On Other Planets Other Resources: • Explain how components of a solar system may be formed • Michigan Teacher Network Resources http://mtn.merit.edu/mcf/SCI.V.4.HS.3.html • Explain how stars produce energy. • • Explain how heavy elements are formed as a result of fusion during supernova explosions; a series of these explosions over time has spread heavy elements randomly throughout the universe. Astronomy Picture of the Day Archive: http://antwrp.gsfc.nasa.gov/apod/archivepix.html • Scope Unit – Solar System, Galaxy and Universe • Pictures from the Hubble Space http://www.stsci.edu/pubinfo/Pictures.html • Periodic Table. • Virtual Sun. http://www.michielb.nl/sun/ • Upton Planetarium • Explain how stars and planets may be formed by the random coalescence (accretion from collisions) of elements or by gravitational attraction. Instruction Assessment Benchmark Question: What star processes are responsible for generating both energy and planetary systems? The only known life in our universe is carbon-based. Carbon has an atomic mass of 12 amu. Focus Question: What happens to mass when hydrogen atoms combine to make helium? Each student will write an essay and answer the following questions: 1. The teacher should review with students the process of nuclear fusion, during which heavier elements are made from lighter ones. One form of fusion involves two protons (hydrogen nuclei) and two neutrons combining to make one helium atom, a process that takes place at very high temperatures in the cores of stars. • By what process could a larger atom-like carbon have formed? • Which two combinations of lighter elements could explain the formation of carbon? Mass of 1 proton = 1.01 amu (atomic mass units). Mass of 1 neutron = 1.01 amu (atomic mass units). Criteria Apprentice Basic Meets Exceeds Accuracy of description Describes fusion as a process that produces energy. Describes fusion as a process that produces energy in stars. Describes fusion as a process that produces energy in stars and forms heavier elements from lighter ones. Describes fusion as a process that produces energy in stars and forms heavier elements from lighter ones with a loss in mass. Accuracy of data Selects mass numbers from the periodic table. Selects a pair of atoms smaller than carbon. Selects one pair of atoms whose atomic masses add up to 12 amu. Selects two different pairs of atoms whose atomic masses add up to 12 amu. Students will look up the atomic mass of helium on the periodic table (He = 4.00 amu). Students will determine the number of hydrogen atoms that combine with two neutrons to make one helium atom. By calculation, students will compare the mass of the two hydrogen atoms and two neutrons to the mass of a single helium atom. Students should answer the question, “Where did that mass go?” Note: Mass is converted to the energy that powers the star 2. Students create an instructional manual for a Build your own Solar System Model, starting from the beginning. 3. Students will make a board game using the Solar System and Solar System formation as a topic Teacher Notes: Origins of the solar system and how we learn about the universe. Scientifically literate students at the high school level should be able to explain the tools of astronomy and the historical contexts of astronomical advances. According to AAAS Project 2061, these explanations may be difficult even for high school students who may "think their predecessors were intellectually and morally inferior or may account for their thoughts and behavior with stereotypes before they understand that past values, beliefs and attitudes were often different from those of today."