STATISTICS 402B Spring 2016 Homework Set#6 1. Using a 2
advertisement

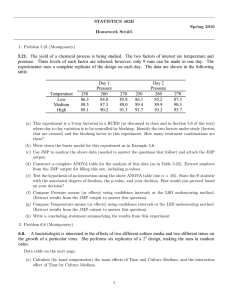
STATISTICS 402B Spring 2016 Homework Set#6 1. Using a 23 factorial, a chemical engineer studied the effects of Temperature (A), catalyst (B), and pH (C) on the yield of a chemical reaction. The levels of each of the variables were (130◦ , 150◦ ), (1%, 2%), and (6.8, 6.9) respectively. A randomized complete block design was used by randomizing the order of the 8 runs within each of 2 successive weeks. The following results have been obtained: Treatment Yield Combination Week 1 Week 2 (1) 60.4 62.1 a 74.4 72.1 b 63.8 65.2 ab 67.3 66.7 c 68.6 65.3 ac 79.9 82.4 bc 68.1 71.3 abc 75.3 77.1 Use JMP to perform the calculations to obtain answers to the following parts using the file chemical reaction.jmp. Obtain the estimates of effects and the sums of squares due to the effects by setting the factors as continuous variables You may re-do the JMP analysis setting the factors as nominal variables and fitting a full factorial to obtain confidence intervals and plots. Remember that Week is a blocking factor and is not part of the factorial treatment structure. (a) Fill out the following table first: Source of Variation Treatment Week Error Total d.f. SS (b) Breakdown the treatment SS in the above anova table into single degree of freedom SS corresponding to each of the effects in this experiment. (c) Perform F-tests for the above effects by completing the anova table to include columns for MS, F, and p-value. What are the effects that are significant at α = .05? (d) Provide estimates of all effects. (e) The significance of these effects can also be tested using appropriate confidence intervals. Use the JMP output to extract the relevant 95% confidence intervals. Provide a table of 95% CI’s (arranged as on page 11 of Note Set #10). (f) Interpret each significant interaction effect(s) using a suitable 2 × 2 table of means and a figure representing simple effects. (These plots and tables are available in the second JMP output.) 2. An experiment is run on a current chemical process in which changes to operating conditions are being studied according to the following table: A: B: C: D: Variable concentration of catalyst(%) concentration of NaOH(%) agitation speed (rpm) temperature (◦ F ) 1 − 5 40 10 150 + 7 45 20 180 The experiment is run as a 24 factorial in a single replication and the 16 runs are carried out in completely random order. The response is an impurity measurement on the resulting chemical product. The following results were obtained: Run Order 2 6 12 4 1 7 14 3 8 10 15 11 16 9 5 13 A − + − + − + − + − + − + − + − + B − − + + − − + + − − + + − − + + C − − − − + + + + − − − − + + + + D − − − − − − − − + + + + + + + + Impurity 0.38 0.40 0.27 0.30 0.58 0.56 0.30 0.32 0.59 0.62 0.53 0.50 0.79 0.75 0.53 0.54 The data are available in the JMP file chemical process.jmp. (a) Use JMP to calculate the sums of squares due to the effects by setting the factors as continuous variables and fitting a full factorial. (b) Follow the method discussed in class (in relation to the analysis of Montgomery Example 6.2) to obtain a normal probability plot of the effects by making a JMP data table out of the parameter estimates. (c) Identify the important effects using the above plot and make a table showing their effects and sums of squares. (d) Use JMP again to fit a full factorial setting the factors as nominal variables. Obtain plots and 2 × 2 tables to interpret any important interaction effects you found above. (Hint: See Effect Details for the Effect you are interested and use LSMeans Plot to get the plot). Interpret the effects as on page 6/7 of Note Set #11. (e) Investigate whether there is a factor whose main effect and all interactions involving it are negligible. If this is so, use design projection to obtain an estimate of error variance. Provide an analysis of variance table as on page 9 of Note Set #11. Identify significant effects based on this. (f) If the current operating conditions are: A=5%,B=40%, C= 10 rpm, D=180◦ F , make recommendations based on conclusions from your analysis to reduce the mean impurity content. Note: Need to present written answers to each part, with calculation shown for parts that you are required to do hand computation. Use the JMP output to obtain numbers for answering other parts. Attach edited JMP output when you use the JMP output to extract numbers as part of the analysis. The JMP data files chemical reaction.jmp and chemical process.jmp are available to download. Due Friday, April 15th, 2016 (turn-in at the beginning of class) 2