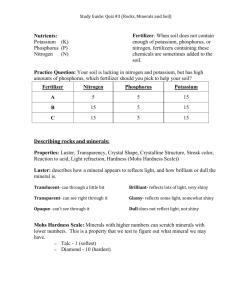
AN ABSTRACT OF THE THESIS OF
advertisement

AN ABSTRACT OF THE THESIS OF Dost Mohammad Ba loch for the degree of Doctor of Philosophy in Crop Science presented on July 31, 1998. Title: Evaluation and Use of a Soil Mineralizable Nitrogen Test to Determine the Fertilizer Nitrogen Needs of Winter Wheat Grown in Western Oregon Abstract approved: Russell S. Karow The assessment of optimum nitrogen (N) fertilizer need for winter wheat (Triticum aestivum L.) is important for economic and environmental sustainability. A comprehensive understanding of fertilizer N requirement depends on estimation of the quantity of N needed by the crop versus that supplied by soil. The objectives of this study were: to assess the potential of using short-term anaerobic incubation test values in developing nitrogen fertilizer recommendations for the region; to evaluate the suitability of a nitrogen balance model for predicting N fertilizer needs of winter wheat; and to document the N uptake patterns of spring and winter wheat. Field experiments were conducted in the Willamette Valley of western Oregon in 1994-1997 and included both on-farm trials and small research plot trials. An array of rotations, crop types and nitrogen fertilizer rates were evaluated. Mineralizable nitrogen in soil samples taken prior to spring fertilization was estimated by a 7-day anaerobic incubation method and results were compared with estimations of soil supplied nitrogen from field experiments. Lab and field estimations were well correlated. A more than four fold increase in soil supplied N values, 20 to 110 kg N III, was observed when mineralizable N test values increased from 14 to 29 mg N kg'. Results indicate that soil mineralizable N values satisfactorily predict approximate soil N availability and that results can be used to adjust fertilizer N requirements in the region. A Feekes growth stage 5 spring soil and tissue test based model was developed and evaluated for predicting the need for additional N fertilizer on winter wheat. Optimum N rates predicted by the model were closely related to N rates required to obtain maximum economic yield. Model validation experiments also gave promising results. Nitrogen uptake patterns for spring and winter wheat were similar. Maximum N uptake for spring wheat was at approximately 1100 accumulated growing GDD, before Feekes 10. The maximum N uptake rate, 0.038 kg N GDD-1, occurred at 750 GDD. Nitrogen uptake in winter wheat was significantly affected by rotations. Maximum N uptake for fertilized winter wheat was at approximately 1400 GDD, also before Feekes 10. The maximum N uptake rate, 0.5 and 0.2 kg N GDD-1, for wheat following clover and oat occurred at 1100 and 1300 GDD, respectively. Evaluation and Use of a Soil Mineralizable Nitrogen Test to Determine the Fertilizer Nitrogen Needs of Winter Wheat Grown in Western Oregon by Dost M. Baloch A THESIS submitted to Oregon State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy Completed July 31, 1998 Commencement June 1999 Doctor of Philosophy thesis of Dost Mohammad Baloch presented on July 31, 1998 APPROVED: Major professor, representing Crop Science Head of Department of Crop and Soil Science Redacted for privacy Dean of Grad e School I understand that my thesis will become part of the permanent collection of Oregon State University libraries. My signature below authorizes release of my thesis to any reader upon request. Redacted for privacy Dost Mohammad Baloch ACKNOWLEDGMENT I would like to express my deep gratitude and sincere appreciation to my advisor, friend and supervisor, Dr. Russell S. Karow, for his generous advice, incredible support, and encouragement through all aspects of the study. His constant assistance and academic advice made it possible to complete this dissertation. It has been a great pleasure and pride to work under his supervision. I will never forget his extreme devotion, dedication and willingness to helping others with smiling face. I am grateful to the members of my committee, Dr. Neil W. Christensen and Dr. John Hart for their availability, interest and incredible guidance. I am also thankful to my minor professor Dr. William Braunworth and graduate representative Dr. Frederick Obermiller for their valuable advice and review of my dissertation. Sincere gratitude to Ernie Marx for not only helping me conduct field experiments, but also discussing social, economical and political issues. Special thanks to Barbara Reed for her cooperation throughout my course of study. I am also thankful to Bruce and Helle Ruddenklau, James Van Leeuwen, Lincoln Volker and Chris Chipman for their help in conducting field experiments on their farms. My appreciation is also extended to Steve Petrie, agronomist, UNOCAL chemical company for grants and for supplying fertilizer. Mark Mellbye, Linn-Benton-Lane field crop extension agent, for his help during on-farm experiments. I am also very appreciative of the research funding provided by the Oregon Department of Agriculture Groundwater Research and Development Grant Program. I owe great thanks to my home friends, Maqsood Hassan Qureshi, Yousaf Hussain Bangash, Sami Molvi, Syed Navid Raja and Atta-a- Kareem for their emotional, material and moral support. Their companionship, compassion, and mutual admiration made possible my stay at Oregon State University. My appreciation from the bottom of my heart goes to Christy M. Haarsma, whose heartfelt support, encouragement and inspiration reinforced my personal desire to complete my degree. Words would never be enough to express my heartfelt gratitude to my affectionate father and loving mother whose love not only for me but for knowledge as well, was the sole inspiration that enabled me to get such an achievement. I am also grateful to my brothers, sisters and all members of my family, whose love, support, and encouragement helped me overcome problems and difficulties, I ever experienced in my life. Above all Thanks 0! Allah, The Allmighty. TABLE OF CONTENTS Page CHAPTER I. INTRODUCTION CHAPTER 2. VALIDATION OF SOIL MINERALIZABLE NITROGEN TEST 1 . . 11 . Abstract 11 Introduction 13 Materials and Methods 21 On-Farm Trials Small-plots Rotation Trials Unfertilized On-farm Mini-plots Laboratory Analysis Anaerobic Incubation 21 23 24 25 26 26 27 Soil Supplied N Mineral N Balance Method Soil Supplied N Unfertilized Plots Results and Discussion 28 Conclusions 36 References 37 CHAPTER 3. A MODEL FOR MAKING FIELD-BASED NITROGEN RECOMMENDATIONS FOR WINTER WHEAT IN WESTERN OREGON. . . . 43 Abstract 43 Introduction 45 Materials and Methods 51 Nitrogen Balance Model On-Farm Trials Small Plot Research Station Trials Model Validation Trial Maximum Economic Yield Equations Soil Analysis Anaerobic Incubation 51 52 55 56 58 59 60 TABLE OF CONTENTS (Continued) Page Results and Discussion Grain Yield Response Determination of Nitrogen Rates for Optimum Grain Yield Model Validation 61 63 69 72 Conclusions 75 Further Recommendations 77 References 78 CHAPTER 4. EVALUATION OF NITROGEN UPTAKE PATTERN IN SPRING AND WINTER WHEAT IN WESTERN OREGON 83 Abstract 83 Introduction 85 Materials and Methods 89 Spring Wheat Rotation Experiment Small Plot Research Station Trials N uptake from Unfertilized Plots Statistical Analysis Results and Discussion Spring wheat Winter wheat 89 91 91 92 94 94 100 Conclusions 105 References 108 CONCLUSIONS 111 BIBLIOGRAPHY 113 APPENDIX 125 LIST OF FIGURES Figure Page 1-1. Soil mineralizable N (closed symbols) and soil residual N (NO3-N and NH4-N, open symbols) taken throughout winter from three rotations in 1994 growing season 29 1-2. Relationship between mineralizable N test values and soil supplied N. Each value is an individual check plots from: on-farm experiment over three years, small research plots over two years, and on-farm mini plots over one year. Soil supplied N values were obtained by two methods (A) N uptake (B) N balance method 33 1-3. Relationship between mineralizable N test values and soil supplied N across four rotations. Each value is an individual check plots from: on-farm experiment over three years, small research plots over two years, and on-farm mini plots over one year. Soil supplied N values were obtained by using N balancing method 35 2-1. Yield response of (a) Clover-wheat (b) Corn-wheat (c) Grass-wheat rotations to fertilizer N rates of on-farm trials for the 1994-96 growing season. 65 2-2. Yield response of clover-wheat and oat-wheat( A) 1996 (B) 1997 of small research plots. 73 2-3. Total biomass, grain yield and protein content as influenced by fertilizer N for wheat following grass in 1996 growing season.. 76 3-1. Comparison of five spring wheat cultivars for biomass accumulation in 1997 growing season 3-2. Comparison of five spring wheat cultivars for nitrogen uptake in 1997 growing season. 96 98 3-3 Average nitrogen uptake and biomass accumulation rates of spring wheat in 1997 growing season 99 3-4 Nitrogen uptake in winter wheat as influenced by different rotations and fertilizer N rates in (A) 1996 and (B) 1997 at Hyslop Farm.. 101 3-5 Nitrogen uptake rate in winter wheat receiving 100 kg N ha -1 as influenced by different rotations in (A) 1996 and (B) 1997 at Hyslop Farm 102 LIST OF FIGURES (Continued) Figure 3-6. Accumulated biomass yield and nitrogen uptake of winter wheat from unfertilized plot during 1996 growing season Page 106 LIST OF TABLES Table Page 1-1. Location, previous crop, variety and soil series for experimental sites 1-2. Ammonium, nitrate and mineralizable nitrogen measured in top 30 cm of soil at spring fertilization in February during 1995-96 growing season 22 30 1-3. Mean values of mineralizable soil N measured in top 30 cm of soil at spring fertilization and after harvesting over three years and rotations 31 2-1. Location, previous crop, variety and soil series for experimental sites. 53 2-2. Assessment of optimum N rates for each rotation using the N balance model 62 2-3. Mean grain yield of on-farm trials for the 1994-96 growing season. 64 2-4. Regression equations for nitrogen response curves of on-farm trials for the 1994-1996 growing season 2-5. Comparison of recommended and actual N rates for maximum economic yield 67 71 2-6. Main yield and protein content for model validation trials conducted during 1996-97 winter wheat growing season 74 3-1. Sampling dates and corresponding Feekes growth stages, and accumulated growing degree days for spring and winter wheat experimental sites 3-2 Accumulative biomass yield and nitrogen uptake of five spring wheat cultivars for 1997 growing season. 90 95 3-3. Biomass yield, nitrogen uptake, and carbon to nitrogen ration of winter wheat of small plot research experiment for 1996 and 1997 growing seasons 104 LIST OF APPENDIX TABLES Table 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 Page Agronomic data of wheat following clover for 1994-95 Agronomic data of wheat following clover for 1995-96 Agronomic data of wheat following corn for 1994-95. Agronomic data of wheat following corn for 1995-96 Agronomic data of wheat following grass for 1994-95 Agronomic data of wheat following grass for 1995-96 Soil test results through winter months for 1994-95 End of season soil test results of wheat following clover End of season soil test results of wheat following corn End of season soil test results of wheat following grass Soil test results of wheat following clover 1995-96 End of season soil test results of wheat following clover Soil test results of wheat following corn 1995-96 End of season soil test results of wheat following corn Soil test results of wheat following grass 1995-96 End of season soil test results of wheat following grass Agronomic data for Hyslop Farm for 1995-96 Agronomic data for Hyslop Farm for 1995-96 Biomass and N uptake of five spring wheat varieties Data from on-farm-mini plots 1995-96 Agronomic data for wheat following corn. Agronomic data for wheat following grass. 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 147 148 148 Dedicated to my father SHAH MIR KHAN EVALUATION AND USE OF A SOIL MINERALIZABLE NITROGEN TEST TO DETERMINE THE FERTILIZER NITROGEN NEEDS OF WINTER WHEAT GROWN IN WESTERN OREGON CHAPTER 1 INTRODUCTION The importance of a quick, reliable soil N mineraliztion test becomes ever greater due to economic and environmental concerns. Currently, recommendations for nitrogen fertilizer are mainly based on crop requirements. It is assumed that the nitrogen mineralized during the growing season is either not important or closely related to the initial soil N content. Not taking account of the soils potential for N mineralization often leads to the excess use of N fertilizer resulting in ground and surface water pollution. The availability of nitrogen is the prerequisite for agroecosystem productivity. On a large scale, the cyclic nature of this essential element provides sufficient supply for plant growth without depleting the soil. On a global basis, within natural and agricultural terrestrial system, soils are the major N reservoirs, containing 2.4 x 1011 tons of N (Stevenson, 1982). Fertilizer addition, dry deposition, biological fixation and precipitation are the main N input sources for soils. From 95 to occurs as a part of organic molecules (Jansson, 99 1971; percent of the nitrogen in a soil Tisdale et al., 1985). This protects it from loss but also leaves it unavailable to plants. In order to be utilized by plants, soil organic N compounds have to be changed to an inorganic form. Conversion of organic N 2 into inorganic N is carried out by soil microorganisms through a process called mineralization. Parnas (1975) described N mineralization as a by-product of microbial activity. Researchers have long been aware that a small percentage of organic N is mineralized in the course of the growing season and can significantly contribute to nitrogen requirements of the crop. Forth and Ellis (1988) reported that in a well-drained mineral soil about two percent of the organic N is mineralized annually. Harper (1984) and Power and Doran (1984) reported that the mineralization process converts from one to three percent of total organic N in a soil to inorganic N annually. Marumoto et al. (1982) argued that microorganisms may provide up to 40 percent of crop N need through mineralization. Soil N mineralization is profoundly influenced by temperature within the range normally encountered under field conditions. Temperature greatly influences the microbial population, substrate composition, nutrient cycling and transport processes in the soil. The majority of soil micro-organisms are mesophllic and prefer moderate temperatures with an optimum activity between 25 °C and 37 °C and a base temperature of 5 °C. Unlike mesophiles, psychrophiles become active at lower temperature and play a significant role in N mineralization during low temperature periods of the year (Jarvis et al., 1996). Since mineralization ceases near the freezing point, the temperatures of greatest interest in soil biology generally occurs in the range of 0 to 35° C. Stanford et al (1973) found that the mineralization rate doubled with each 10 °C increase in temperature up to 35 °C. Soil water availability and distribution are prominent factors regulating N mineralization in 3 soils. The water status of soil affects biological activities and the products of microbial activity by controlling diffusion and mass flow. Linn and Doran (1984) found that optimum N mineralization occurred when 60 percent of pore space was water filled. Drying the soil below the optimum moisture content reduces mineralization and ultimately affects the amount of available N in the soil. Soil drying to -100 kPa increased subsequent N mineralization under optimum conditions (Seneviratne and Wild, 1984). Stanford and Epstein (1974) reported that optimum net mineralization rates have been achieved between -0.33 and -0.1 bar where water occupied 80 to 90 percent of the pore space. It is well known that drying and wetting increases N mineralization (Lund and Goksoyr, 1980; Orchard and Cook, 1983; Sparling and Ross, 1988), but the mechanisms involved are poorly understood. Marumoto et al. (1982) stated that physical disruption or increased accessibility of degradable organic materials, especially readily available materials from freshly killed biomass cells, are the main cause of the flush of mineral N after drying and wetting. Soil microbial biomass (SMB) is a potential source of labile N and constitutes a significant proportion of the total soil N which remains constant throughout the year (Holmes, 1994). In spite of the fact that N mineralization can occur in deeper soil horizons (Casman and Munns, 1980; Hadas et al., 1986), most research has been limited to the upper layers. Soil layers vary in temperature, moisture, bulk density, aeration and organic matter content, all of which may affect N mineralization at different depths. Federer (1983) examined the dependence of mineralization and nitrification on horizons through a soil profile by incubating soil in situ in buried bags. He reported that net mineralization of 4 nitrogen decreased with depth. Change in crop sequence changes soil temperature, soil moisture, crop rooting and residue inputs. Crops grown in rotation often produce more and higher quality plant dry matter than those grown in monoculture (Copeland and Crookston, 1992). Crop rotation helps balance the carbon-nitrogen ratio of the organic matter by adding different C:N ratio residues to the soil. Cereal crops grown after a legume often have a higher yield as compared to a non-legume crop (Wani et al., 1990). This is because legume residues generally have a low C:N ratio and large fraction of readily available N. Thicke et al. (1993) found that the decomposition of incorporated legume residues can a have significant, but extremely variable, impact on total N mineralization. McKenney et al. (1995) concluded that legume residues can provide a definite advantage compared with grass and corn residue in term of N conservation depending on the aeration status of the soil. Legume residues not only provide a great source of readily available N to other crops (Azam, 1990), but can also increase long-term soil fertility (Palm and Sanchez, 1991). This increase is mainly because of the conversion of a portion of biologically-fixed N into the stable humus form (Azam et al., 1993). In addition to the enhancement of N fertility, legumes also affect soil properties such as water holding capacity. cation exchange capacity, buffer capacity, soil porosity and infiltration (Cook, 1988; Fyson and Oaks, 1990). Many legumes have deep root systems which allow exudates to exist at greater soil depth. These exudates may serve as solubilization or chelating agents of plant nutrients fixed in the unavailable form. 5 Choice of cropping and management practices used within a given crop production system play an important role in the utilization of soil N. Physical disturbance of the soil surface horizon with tillage greatly affects the soil micro environment (Doran and Power, 1983). Changes in tillage and residue management systems directly affect the temperature and water status of the soil. Tillage also increases the accessibility of organic N to the microbes. Breaking up of soil aggregates and exposing previously protected organic N by tillage commonly stimulates short term N mineralization (Gupta and Germida, 1988). On the other hand, no-till soils tend to be wetter, have different temperature regimes, and are more compact than conventionally tilled soils and favor microbial activity. Dalai (1989) compared the effect of 13-years of conventional tillage vs. no-tillage, crop residue retained vs. burned, and no fertilizer N vs. N fertilizer. His results indicate that total N and biologically mineralizable N in the soil can be increased by no-tillage and crop residue retention with a moderate rate of N fertilizer (23 kg ha4 year-1). Though factors and conditions determining N mineralization have been intensively investigated, the development of a quick, easy and reliable soil N mineralization test has not been accomplished. Several methods for quantifying N mineralization have been proposed including, laboratory, field and modeling techniques. Each method has its own unique criteria of implementation with advantages and disadvantages. The aim of all these methods has been to provide a more precise basis for estimating N mineralization potential of soil so that better N fertilizer recommendations can be made. Because of the complex nature of N mineralization processes, none of the procedures have proven to be totally adequate. 6 A great number of laboratory methods have been suggested including aerobic and anaerobic incubations. The aim of such incubations has been to quantify the available organic N pool for microbial decomposition under optimal conditions. The advantages of these procedures are that they are relatively quick, offer control over certain limiting factors and are relatively inexpensive. But the correlation between laboratory indices and field studies is very poor because the laboratory methods do not include environmental factors that regulate mineralization rate. Although good correlations often exist between N released during incubation and uptake in green house studies (Stanford, 1982), incubation test results do not necessarily reflect the actual N mineralization capacity of the soil (Fox and Piekielek, 1984). Field measurements of N mineralization are the most time consuming and expensive way of estimating N mineralization. But they are also the most promising technique which provide a degree of reality that may be difficult to achieve with either laboratory procedures or computer simulations. Field approaches have the potential of including the effect of regulatory factors on mineralization rate. Hence, assessment of net mineralization under field conditions is the best and most accurate way of assessing the N supplying power of the soil (Binkley and Hart, 1989). Numerous filed approaches to estimate mineralization have been developed in recent years including nonfertilized cropped plots, covered fallow plots, uncovered fallow plots, mineralization bags and PVC tubes. The Pacific Northwest has very unique climatic conditions with humid winters and dry summers. Most of the precipitation, 60 to 70 percent annually, occurs from the first 7 week of October to the last week of March. Topography varies from nearly level valleys to steeply sloping uplands. These situations make it very easy for soil nitrogen to disappear from the system and very difficult for researchers to trace mineralizable N as well. Winter wheat is grown in rotation with grasses, legumes, grains and vegetables in western Oregon. The residual N level differs significantly in these rotations and is strongly influenced by weather pattern and specific site. Growers in the region are interested in knowing how much residual N will be available form the proceeding crop and how best to adjust their fertilizer rates to optimize yield while reducing risk of nitrogen pollution and losses. The goal of this research was to establish the specific nitrogen status and requirement of winter wheat crops grown in various rotations in western Oregon. The main objectives of the study were: 1 To investigate the use of soil testing and tissue analysis as a means of predicting nitrogen fertilizer needs of winter wheat. 2 - To measure the effect of crop rotation on residual soil nitrogen level and yield of a subsequent wheat crop. 3 To examine the dynamics of spring and winter wheat N uptake. 8 References Azam, F. 1990. Comparative effect of organic and inorganic nitrogen sources applied to a flooded soil on rice yield and availability. Plant Soil. 125:255-262. Azam, F., F. W. Simmons, and R. L. Mulvaney. 1993. Mineralization of plant residues and its interaction with native soil N availability. Soil Biol. Biochem. 25:1787­ 1792. Binkley, D., and S. C. Hart. 1989. The components of nitrogen availability assessment in forest soils. Adv. Soil Sci. 10:57-112. Cassman, K. G., and D. N. Munns. 1980. Nitrogen mineralization as affected by moisture, temperature and depth. Soil Sci. Soc. Am. J. 44:1233-1237. Cook, R. J. 1988. Biological control and holistic plant-health care in agriculture. Am. J. Alt. Agri. 3:51-62. Copeland, P. J., and R. K. Crookston. 1992. Crop sequence affects nutrient composition of corn and soybean growth under high fertility. Agron. J. 84:503-509. Dalal, R. C. 1989. Long-term effect of no-tillage, crop residue, and nitrogen application on properties of a vertisol. Soil. Sci. Soc. Am. J. 53:1511-1515. Doran J. W., and J. F. Power. 1983. Effect of tillage on the nitrogen cycling in corn and wheat production. In nutrient cycling in agriculture ecosystem. University of Georgia. Athens. Special publication No. 23:441-455. Federer, C. A. 1983. Nitrogen mineralization and nitrification: Depth variation in four New England forest soils. Soil Sci. Am. J. 47:1008-1014. Forth, D. H., and B. G. Ellis. 1988. The soil nitrogen cycle. In Soil Fertility. John Wiley & Sons. Ins. p. 69-73. Fox, R.H., and W. P. Piekielek. 1984. Relationship between =aerobically mineralized nitrogen, chemical indexes, and nitrogen availability to corn. Soil Sci. Soc. Am. J. 48:1087-1090 Fyson, A., and A. Oaks. 1990. Growth proportion of maize by legume soils. Plant Soil. 122:259-266. 9 Gupta, V. R., and J. J. Germinde, 1988. Distribution of microbial biomass and its activity in different soil aggregate size classes as affected by cultivation. Soil Biol. Biochem. 17:517-523. Hadas, A., S. Feigenbaum., A. Feigin, and R. Portnoy. 1986. Nitrogen mineralization rate in profile of differently managed soil types. Soil Sci. Am. J. 50: 314-319 Harper, J. E. 1984. Uptake of Organic Nitrogen Forms by Roots and Leaves. In R. D. Hauck(ed.) Nitrogen in Crop Production. Am. Soc. Agron. Madison, WI. Holmes, W. E. 1994. Soil microbial biomass dynamics and net mineralization in northern hardwood ecosystems. Soil. Sci. Soc. Am. J. 58:238-243. Jansson, S. L. 1971. Use of15N in studies of soil nitrogen. Soil Biol. Biochm. 2:129-166. Jarvis, S. C., A. S. Elizabeth., M. A. Shepherd,. and D. S. Powlson. 1996. Nitrogen mineralization in temperate agriculture soils: Processes and measurement. advances in agronomy. Vol. 57. Acadamic Press. pp 187-230. Linn D. M., and W. Doran. 1984. Effect of water filled pore and space on CO2 and NO2 production in tilled and no-tilled soils. Soil Sci. Soc. Am. J. 48:1272-1276. Lund, V., and B. Goksoyr. 1980. Effects of water fluctuations on microbial mass and activities. Micro. Eco. 6:115-123. Marumoto, T., J. P. Anderson, and K. H. Domsch. 1982. Mineralization of nutrients from soil microbial biomass. Soil Biol. Biochem. 14:469-475. McKenney, D. J., S. W. Wang., C. F. Drury., and W. I. Findlay. 1995. Denitrification, immobilization, and mineralization in nitrate limited and non limited residueamended soil. Soil Sci. Soc. Am. J. 59:118-124. Orchard, V. A., and Cook, F. J. 1988. Relationship between soil respiration and soil moisture. Soil Biol. Biochem. 15:447-453. Palm, C. A., and Sanchez, P. A. 1991. Nitrogen release from some tropical legumes as affected by lignin contents. Soil Biol. Biochem. 23:83-88. Parnas, H. 1975. Model for decomposition of organic material by microorganisms. Soil Biol. Bioch. 7:161-169. Power. J. F., and J. W. Doran. 1984. Nitrogen Use in Organic Farming. In R. D. Hauck(ed.) Nitrogen in Crop Production. Am. Soc. Agron. Madison, WI. 10 Seneviratne, R., and A. Wild. 1984. Effect of mild drying on the mineralization of soil nitrogen. Plant and Soil. 84:175-179. Sparling, G. P., and D. J. Ross. 1988. Microbial contributions to the increased nitrogen mineralization after air-drying of soils. Plant and Soil. 105:163-167. Stanford, G. 1982. Assessment of soil N availability. p. 651-688. in F. J. Stevenson (ed). Nitrogen in agriculture soils. Agron. Monogr. 22. ASA. CSSA, and SSSA, Madison, WI. Stanford, G., and E. Epstein. 1974. Nitrogen mineralization-water relations in the soils. Soil Sci. Soc. Am. Proc. 38:103-107. Stevenson, F. J. 1982. Origin and distribution of nitrogen in soils. In Nitrogen in Agricultural Soils. ASA, CSSA, SSSA, Madison. 1-42. Thicke, F. E., M. P. Russelle,. 0. B. Hesterman, and C. C. Sheaffer. 1993. Soil nitrogen mineralization indexes and corn response in crop rotation. Soil Science. 156:322­ 334. Tisdale, S. L., W. L. Nelson, and J. D. Beaton. 1985. Soil fertility and fertilizers. New York. Macmillan Publishing Company. Wani, S. P., W.B. McGill, and L.A. Robertson. 1991. Soil N dynamics and N yield of barley grown on Breton loam using biological fixation or fertilizer. Bio. Ferti. Soils. 12:1018. 11 CHAPTER 2 VALIDATION OF SOIL MINERALIZABLE NITROGEN TEST Abstract Precise fertilizer N recommendations for a crop require an accurate assessment of soil N availability. Several indices have been identified to estimate soil N availability. Biological indices provide a sound relative measure of the soil N availability since they involve microbial processes similar to those active under field conditions. However, in order to check the validity of these tests, it is imperative that these indices be compared with values for soil supplied nitrogen obtained under field conditions. Our objective was to investigate the utility of short-term anaerobic incubation as a tool to be used in making N fertilizer recommendations for the region. Data used in this study were acquired from three sets of winter wheat (Triticum aestivum L.) nitrogen response experiments: (1) on-farm rotation trials, (2) small plot rotation trials, and (3) unfertilized on-farm mini-plots. On-farm experiments were conducted in growers' fields at three different locations across the Willamette Valley of western Oregon during 1994­ 1996 growing seasons. Three rotations - wheat following corn, wheat following clover, and wheat following grass were considered. Small plot rotation trials were conducted during 1996-1997 growing seasons and two rotations - wheat following oat and wheat following clover were used. Unfertilized-mini plots were established during the 1995-96 12 growing season in 11 grower fields by leaving an unfertilized, randomly selected 15m X 15m area. Mineralizable nitrogen in soil samples taken prior to spring fertilization was estimated by an anaerobic incubation method and results were compared with estimation of soil supplied nitrogen from field experiments. Soil supplied nitrogen was estimated by two different methods; nitrogen balance and aboveground N uptake of check plots. Soil mineralizable nitrogen estimated by the 7-day anaerobic incubation method correlated well with field-measured N availability. The highest correlation for soil mineralizable N test values was observed when soil supplied N was measured by the N budgeting method ( r2 =0.78). However, the correlation with soil supplied N estimated by N uptake of check plots was also high (r2 = 0.69). A linear relationship was found between soil mineralizable N test results and soil supplied N. A more than four fold increase in soil supplied N values, 20 to 110 kg N 11-1, was observed when the soil mineralizable N test values increased from 14 to 29 mg N kg-1. No consistent rotation effect was observed for wheat following clover or corn while rotation effects were obvious for wheat following oat and wheat following grass. Wheat following oat had very low soil mineralizable N test values while wheat following grass had comparatively high soil mineralizable N test values. Our results indicate that soil mineralizable N test values estimated by the 7-day anaerobic incubation method satisfactorily predict approximate soil N availability in western Oregon soils and that results can be used to adjust fertilizer N requirements in western Oregon. 13 Introduction One of the primary goals of current soil fertility research is to improve the efficiency of (N) fertilization, thereby improving the profit margin and reducing risk of ground and surface water contamination by NO3-N. The potential for lodging, diseases, yield losses and N contamination of surface and ground water increases as excessive N fertilizer rates are applied. On the other hand, low rates of N fertilizer can cause considerable yield reduction. Determination of the precise fertilizer N requirement of the crop depends on an estimation of the quantity of N needed by the crop and an estimate of both inorganic and organic soil N sources (Rice and Havlin, 1994). Soil mineral N (NH4-N + NO3-N) testing before spring fertilization has been proven a useful tool for adjusting N fertilizer requirements in the semi-arid and semihumid regions of the U.S. (Maples et al. 1977; Westfall, 1984; Rauschkolb et al., 1984; Cox, 1985). A soil nitrate test before planting appears to be well related to the crop N needs in sub-humid areas in the western US and Canada (Dahnke and Johnson, 1990). Low annual precipitation in these regions usually limits leaching or denitrification of profile NO3-N, resulting in significant carryover of NO3-N ( Bundy and Malone, 1988). Despite complications such as mobility, leaching, and nitrification, soil tests for residual NO3-N have been found to be useful in determining N fertilizer needs of crops (Gelderman et al., 1988). In a study conducted on the Eastern Shore of Maryland, Meisinger et al. (1987) found that the N requirement of a wheat crop could be effectively adjusted by measuring residual spring N status of the soil. 14 In the humid regions of United States, including the Pacific Northwest, the amount of mineral N at the time of spring fertilization has generally not been taken into account when adjusting N fertilizer needs for winter wheat. This is because heavy precipitation during the winter months causes losses of soil NO3-N by denitrification and leaching. Ower (1980) reported that the estimated variable losses of nitrogen from wheat fields within watershed boundaries in western Oregon were 14 to 63 kg N haTI. This typically results in low and uniform mineral N level at the time of spring fertilizeration (Roth, 1987). In a study carried out in western Oregon, Sebastian (1995) found minimal amounts of soil mineral N (NH4-N and NO3-N), 5 to 24 kg N ha-1, in the upper 60 cm of soil at the time of spring application. He suggested that these amounts were not useful in predicting N fertilizer requirements. Broadbent and Carlton (1978) and Sarrantonio and Scott (1988) reported that the NO3-N concentration in soil after winter losses was only 5 to 10 mg N kg-1 . But other researchers have shown that measuring pre-plant soil profile NO3-N in humid climates can be useful in adjusting crop N needs (Bock and Kelly, 1992). In humid areas, researchers have reconfirmed that substantial amounts of NO3-N can remain in medium-to fine-texture soils during the winter period and can contribute substantial amounts of N to subsequent crops (Roth and Fox, 1990; Liang et al., 1991). The cool, humid conditions during winter favor the decomposition of crop residues and, as temperatures increase during the early spring, mineralization of organic N can supply a significant portion of the total N requirement for subsequent crops. Therefore, 15 assessment of mineralizable N at the beginning of the growing season could be helpful in improving fertilizer N recommendations. Conversion of organic N into inorganic N is carried out by soil microorganisms. Researchers have long been aware that a small percentage of organic N is mineralized in the course of the growing season and can significantly contribute to nitrogen requirements of the crop (Foth and Ellis,1988; Harper, 1984; Power and Doran, 1984). Nitrogen mineralization has been shown to provide 3 to 100 percent of the N-nutritional requirements of non-legume crops (Smith et al., 1993). Marumoto et al. (1982) argued that microorganisms may provide up to 40 percent of crop N need through mineralization. Biological immobilization of soil inorganic N occurs simultaneously with mineralization. A significant portion of mineralized N is immediately assimilated by soil microbial biomass and transformed into organic cell constituents (Mary and Recous, 1994). During mineralization-immobilization, soil microbial biomass itself temporarily serves as source and sink of easily mineralizable nitrogen (Smith, 1994). Soil microbial biomass predominantly assimilates NH4-N during the immobilization process, but in the absence of N114-N, may use NO3 -N as an energy source ( Recous et al., 1988; Azam and Malik, 1986). The difference between gross rates of mineralization and immobilization is net mineralization. Net mineralization is strongly dependent on the C:N ratio of organic matter (Whitmore and Groot, 1994; Neve et at., 1994). The amount of N returned to the soil by crop residues depends on the quality and quantity of the substrate. Crops grown in rotation often produce more and higher quality plant dry matter than those grown in monoculture (Copeland and Crookston, 1992; Collins et al., 1992). 16 Change in crop sequence changes soil temperature, soil moisture, crop rooting and residue inputs. Fertilizer N requirement for optimum grain yield is often reduced in rotation compared to monoculture (Franzluebbers et al., 1994). Cereal crops grown after a legume often have a higher yield as compared to those grown after a non-legume crop (Wani et al., 1991). This is because legume residues generally have a low C:N ratio and large fraction of readily available N. McKenney et al. (1995) concluded that legume residues can provide a definite advantage compared with grass and corn residue in term of N conservation depending on the aeration status of the soil. Legume residues not only provide a great source of readily available N to other crops (Azam, 1990), but can also increase long-term soil fertility (Palm and Sanchez, 1991). This increase is mainly because of the conversion of a portion of biologically-fixed N into the stable humus form (Azam et al., 1993). Being a biological process, N mineralization is highly affected by complex interactions among biological, chemical and physical components of the soil and environmental factors (Loiseau et al., 1994; Hassink, 1994; Stanford and Smith 1972; Linn and Doran, 1984; Sparling and Ross, 1988; Ellen and Bettany, 1992). Though factors and conditions determining N mineralization have been intensively investigated, the development of a quick, easy and reliable soil N supply predicting method has still been unsolved. Several methods for quantifying N mineralization have been proposed including laboratory (Keeney, 1982; Gianello and Bremner, 1986; Aiman, 1992; McTaggart and Smith,1993), field (Eno, 1960; Magdoff, 1991; Qian et al., 1993), and modeling (Stanford and Smith, 1972; Campbell et al., 1988; Jones, 1984; Beauchamp, 1986; Houot et al., 17 1989) techniques. Each method has its own unique criterion of implementation with advantages and disadvantages. The aim of all these methods has been to provide a more precise basis for estimating N mineralization capability of soil so that better N fertilizer recommendations can be made. There are two groups of N mineralization indices widely used to provide reference values for soil N mineralization capacities of soil - chemical indices and biological indices. A rapid, convenient and relatively inexpensive way of estimating N release from labile soil organic matter is through use of chemical extractants. It is assumed that the amount of N extracted from a soil sample during the procedure will represent the actual N release to a plant during the growing season. Unfortunately, mineralizable N determined through chemical extractants is not well correlated to N uptake estimated from field experiments. McTaggart and Smith (1993) found that chemical extraction methods did not predict changes due to the recent addition of crop residue. Bremner (1965) and Serna and Pomares (1992) argued that N release by chemical indices can not represent the same fraction(s) of N that would be released in situ by microorganisms. Many chemical extractants such as strong acids or bases, neutral salts and water are being used to assess N mineralization in laboratory procedures. These procedures also vary in extractant concentration and extraction time and temperature. Since living organisms are used in biological incubation methods, such methods are assumed to be more reliable then chemical tests. Biological methods supply a satisfactory measure of soil N potential but are laborious and time-consuming (Keeney, 1982). Incubation techniques may be either long-term or short-term. Short-term incubations are 18 done under aerobic or anaerobic conditions, long-term generally under aerobic. The advantage of short-term procedures are that they are relatively quick, offer control over certain limiting factors and are relatively inexpensive. A short-term anaerobic incubation method described by Keeney (1982) is the most widely used laboratory technique to assess N mineralization capacity of soils. In this method, initial NH4-N content of the soil is determined. Distilled water is added to each sample so that the soil is completely saturated. Samples are covered tightly and incubated for 7 days at 40 °C in an incubator. Final N114-N content of the extract solution is determined from incubated samples. Initial NH4-N values are subtracted from the final values to obtain the N amount mineralized during the 7 day incubation period. Rice and Havlin (1994) recommended use of this method. Wilson et al. (1994) used the method proposed by Keeney (1982) to estimate N mineralization potential of soils and compared the values with a chemical extraction method and found good correlation. In contrast, Fox and Piekielek (1984) found a very poor correlation between mineralizable N values obtained from incubation tests and N mineralized in the field. Biological indices are often highly correlated with greenhouse N uptake. In both cases, measurements are being made under controlled conditions. Although good correlation often exists between N released during incubation and uptake in greenhouse studies (Stanford, 1982), incubation tests results do not necessarily reflect the actual N mineralization capacity of a soil (Fox and Piekielek, 1984). Michrina et al. (1981) found no correlation between relative N uptake in a greenhouse study and relative N uptake in the field. The best way to check the validity of N mineralized through chemical or 19 biological tests is to compare the results with N uptake by plants in the field. The feasibility of N mineralization indices to predict N-suppling capacity of soil has not been well tested under field conditions (Fox and Piekielek, 1978; Thicke et al., 1993). Binldey and Hart (1989) stated that assessment of net mineralization under field conditions is the best and most accurate way of assessing the N supplying power of the soil, since environmental effects are included in the assessment. Using a crop as a sink for N released by soil microbe decomposition of soil organic matter and coupling the results with measurements of mineral N in the soil profile is one of the simplest and the most effective ways of measuring net mineralization. The advantage of this approach is that the study can be carried out without alteration of the system and yet this method also takes into account the environmental and soil factors which regulate N mineralization in the soil. This is also a very labor intensive method and requires a series of plant and soil analyses. Rice and Havlin (1994) argued that plant sampling is the most precise way of estimating N mineralization and is the standard by which other procedures should be compared. Rees et al. (1994) argued that determining plant N uptake and at the same time measuring changes in the pool of soil N and losses from the plant/soil system is the most appropriate way of measuring net mineralization. Net mineralization is simply estimated by the difference between total N uptake and the change in inorganic N in the soil profile over time: N min = (N2 - NO+ Nup [1] where N1 and N2 are the amount of inorganic N in the soil at the beginning and end of the season and Nup is total plant N uptake. In nonfertilized cropped plots, a net mineralization 20 rate is usually high because plant roots compete with microbial populations for mineral N and effectively remove N from the soil and reduce the risk of immobilization. Wang and Bakken (1989) conducted an experiment to examine the effect of spatial distribution of N- rich and N-poor plant residue on N mineralization. They found that in a N-poor plot, net mineralization rates were highest. They argued that it is because plants more effectively competed with microbes for mineral N. Jarvis et al. (1996) argued that this method carefully be considered during the interpretation of the data. Potential problems include: (1) this approach accounts for only above ground biomass while, roots, stubble and senesced leaves may contain a significant portion of N, (2) there may be significant losses through leaching, denitrification or volatilization, (3) the impact of plant exudates on microbial population and on their activities is unknown, and (4) the information is only applicable for a specific soil/crop/environment combination. The objective of this study was to assess the potential of using an anaerobic incubation test value to adjust N fertilizer recommendations in the Willamette Valley of western Oregon. Soil mineralizabe N test values were obtained by using a modified anaerobic incubation method proposed by Keeney (1982), while N supplying capacities were determined through N balancing and measuring N uptake of unfertilized plots. 21 Materials and Methods Data were obtained from unfertilized plots from three sets of nitrogen response experiments (1) on-farm trials, (2) small plot rotation trials and, (3) unfertilized on-farm mini-plots. On-Farm Trials On-farm trials were conducted in growers' fields at three different locations across the Willamette Valley of western Oregon during 1994-1996 growing seasons. The 1993­ 94 experiment was part of fellow graduate student Kevin Sebastian's (1995) MS Thesis. Each site of this experiment represented a crop rotation system that is commonly used in the Willamette Valley. Rotations considered were soft white winter wheat following grass seed, sweet corn or a legume. The experimental design was a randomized complete block with three replications at each site. Plot size varied site-to-site depending on size of machinery used by the grower. An average 90m X 10m plot size was used. Nitrogen fertilizer was applied as urea (45-0-0) at approximately Feekes 5 in one application. Four treatments, including a check receiving no nitrogen, were used at each site during the 1993-94 and 1994-95 growing seasons. At corn and legume rotation sites, N was applied at the rate of 0, 56, 112, and 168 kg N ha-1. Since less residual nitrogen was expected for wheat following grass in rotation, slightly higher rates- 0, 67, 134, and 201 kg N ha'­ were used. During the 1995-96 growing season, five N rates - 0, 56, 112, 168 and 224 kg N ha-1- were used in all three rotations. The location, soil series, cropping history and wheat varieties used in the experiments are listed in Table 1-1. 22 Table 1-1: Location, previous crop, variety and soil series for experimental sites Grower County/City Previous crop Wheat variety 1994 Jones Polk/Amity Clover Madsen Amity Fine-silty, mixed mesic, Argiaquic Xeric Argialbolls Volker Benton/Monroe Corn Gene Malabon Fine, mixed, mesic Pachic Ultic Argixerolls Van Leeuwen Linn/Halsey Tall Fescue Gene Malabon Fine, mixed, mesic Pachic Ultic Argixerolls 1995 Ruddenklau Polk/Amity Clover Gene Amity Fine-silty, mixed mesic, Argiaquic Xeric Argialbolls Volker Benton/Monroe Corn Gene Chehallis Fine-silty, mixed, mesic Cumulic Ultic Haploxerolls Van Leeuwen Linn/Halsey Tall Fescue Stephens Woodburn Fine-silty, mixed, mesic Aquultic Argixerolls 1996 Ruddenldau Polk/Amity Clover Gene Amity Fine-silty, mixed mesic, Argiaquic Xeric Argialbolls Volker Benton/Monroe Corn Gene Malabon Fine, mixed, mesic Pachic Ultic Argixerolls Chipman Benton/Albuny Perennial Raygrass Madsen Willamette Fine-silty, mixed mesic Ultic Haploxeralfs Mulkey Polk/Monmouth Annual Raygrass Madsen Willamette Fine-silty, mixed mesic Ultic Haploxeralfs Jones Benton/Corvallis Corn Stephens Chehallis Fine-silty, mixed, mesic Cumulic Ultic Haploxerolls Soil Series 1997 23 Samples were collected at approximately one month intervals from each site during the 1993-94 and 1994-95 growing seasons. Samples were taken at four depths- 0-30, 30­ 60, 60-90 and 90-120 cm. A single composite sample representing the entire plot area was used prior to spring fertilization. Approximately ten cores were taken to make this composite sample. Analysis of soil N data from the two growing seasons indicated that a 30 cm depth sample was adequate to assess soil N status (data not shown). During the 1995-96 growing season, pre-fertilization soil samples were collected from individual plots to a depth of 30 cm. Each sample was a composite of approximately 10 cores. Intensive soil sampling was performed just after harvest at each site on each plot in all years. To calculate total N uptake, plant tissue samples were taken at Feekes 5 (prior to fertilizer application) and at maturity. At Feekes 5, ten representative samples of 30 cm of row were collected from each plot by cutting the plants at soil level. Whole plant samples were weighed, ground and analyzed for nitrogen content. At maturity, nine one-meter row sections were cut at soil level from each plot to determine dry matter. Heads from plants were removed and threshed separately. Representative subsampling was performed for each sample. Subsamples of grain, straw and chaff were analyzed for nitrogen content. Small-plot Rotation Trials The small plot rotation trials were established during the 1993-94 growing season. The intent was to continue these trials over an extended period of time. The experiment was arranged as a spilt plot design with four replications having rotation as main plots and fertilizer treatments as sub-plots. Rotation plots were 6.4m X 45m in size, while N 24 fertilizer sub plots were 6.4m X 9m. Five fertilizer treatments - 0, 50, 100, 150 and 200 kg N - were used. Rotations included winter wheat following clover and winter wheat following oat. The site had been fallow in 1993. Crimson clover was planted on all plots in fall 1994. In spring 1995, two plots per replication were fallowed, one was planted to oat and the fourth was left in clover. Clover and oat were allowed to mature, were harvested and crop residue was incorporated. Wheat was planted on the clover and oat stubble plots in fall 1995, crimson clover on one of the fallowed plots to establish the next rotation cycle. Data were collected for 1995-96 and 1996-97 growing seasons. Soil samples were collected pre-plant and post-harvest. An intensive pre-plant sampling was done in fall 1995 by taking soil at 30 cm increments to a depth of 150 cm from individual plots. This was done to assess the residual nitrogen status of the experimental site. Post-harvest sampling was performed in 0, 150 and 200 kg N treatment plots. Samples were analyzed for ammonium and nitrate concentrations. Aboveground plant tissue samples were taken at both Feekes 5 and at maturity for dry matter yield and nitrogen uptake. At maturity, four aboveground plant samples of 1.5 meter of row were cut from drill strips in each sub-plot. Heads were cut, threshed and weighed. Grain and straw were analyzed separately for their nitrogen content. Unfertilized On-farm Mini-plots Unfertilized-mini plots were specifically designed to determine the correlation between the lab incubation test for mineralizable N and the amount of nitrogen released in the soil through the growing season. This study was done during the 1995-96 growing 25 season with the cooperation of growers and county extension agents. Growers were asked to randomly select an area of 15m X 15m in one of their fields and leave it unfertilized. Soil samples were taken to a 30 cm depth at Feekes 5 and just after harvesting. Soil samples were analyzed for ammonium, nitrate and mineralizable N. Aboveground plant tissue samples were taken at Feekes 5 and just before harvesting from one 0.90 m of row. Tissue samples were analyzed for nitrogen content. Laboratory Analysis Soil and plant tissue samples were analyzed in the Central Analytic lab (CAL) at Oregon State University, Corvallis, Oregon. Plant samples were dried at 70 °C in a forced air oven, weighed and ground in Wiley mill to pass 1 mm mesh. The N content of wheat grain and straw was determined by Leco CNS 2000 (Leco corporation, St. Joseph, MI) combustion analysis. Soil inorganic N (NH4-N and NO3-N) was determined using the modified KC1 extraction method described by Keeney and Nelson (1982). Twenty-gram soil samples were placed in 250 mL extracting bottles and 75 ml of extracting solution (2 MKCI) was added. Vessels were shaken on a mechanical shaker for one hour. The extraction solution was filtered through Whitman No. 42 filter paper. The NH4-N and NO3-N content of the extract was determined with an ALPKEM rapid flow analyzer (RF-300) which complexes NI-14-N with salicylate to form indophenol blue. This color was intensified with sodium nitroprusside and measured at a wavelength of 660 nm. The NO3-N concentration were determined with the same equipment used for NH4-N analysis by reducing nitrate via a 26 cadmium reactor and complexing the NO3-N with sulfanilamide and N-(1-Napthyl)­ ethylenediamine dihydorchlwide to form a red-purple color. The color intensity was measured at a wavelength of 540 nm. Anaerobic incubation Soil mineralizable N was determined using a short-term anaerobic incubation method described by Keeney (1982), slightly modified by increasing the sample size. Through use of a sample splitter, a 20 g soil sample was obtained and placed in a 250 ml extraction bottle. Fifty mL of distilled water was added to each bottle so that the soil become completely saturated. Bottles were made air tight by putting a plastic cover under their lids. Samples were placed in an incubator for 7 days (168 h) at 40 °C plus or minus 0.5 °C. After incubation, samples were carefully removed from the incubator and 50.0 mL of KCl was added. Vessels were shaken on a mechanical shaker for one hour. The extraction solution was filtered through Whitman No. 42 filter paper. Final NH4-N content of the extract solution was determined from incubated samples. Initial Nat-N values are subtracted from the final values to obtain the amount of N mineralized. Soil supplied N Mineral N Balance Method The net amount of N mineralized under field conditions was estimated through use of a equation proposed by Cabrera and Kissel (1988) modified to exclude root fractions. N = (N2-N1) + (Nup2-Nup1) [2] where N1 and N2 are the total amount of soil inorganic N (NH4-N + NO3-N) at Feekes 5 and the end of the season, respectively, and N p1 and Nup2 are plant N uptake at 27 Feekes 5 and at maturity, receptively. It was assumed that N gain through atmospheric fixation and N losses through denitrifiction and leaching were minimal given the environmental situation of the region. Soil Supplied N-unfertilized Plots Nitrogen uptake values for aboveground tissue were obtained from unfertilized plots and soil N availability was estimated as the N taken up by the crop during the growing season. It was assumed that plants exhibited maximal N uptake and that N losses from soil during the growing season were minimal. 28 Results and Discussion Measurements of soil mineralizable N (SMN) on soil samples taken from the upper 30 cm of soil consistently showed a drop in SMN levels from fall to mid-winter but showed little variation from late December to early February (Fig. 1-1). It was also observed that mineral N (NH4-N and NO3-N) concentration in the soil at the time of spring fertilization was minimal and uniform through all rotations (Fig. 1-1). This is likely because heavy precipitation leached NO3-N and cold weather during winter months slowed down decomposition of previous crop residue. There was no significant difference for mineralizable N values among individual plots at pre-fertilization sampling within each rotation (Table 1-2). There were, however, differences among rotations. Wheat following grass had a higher SMN compared to wheat following clover, 33.7 and 16.5 mg ke, respectively (Table 1-2). Similar results were observed for soil samples taken at the end of season for all rotations (Table 1-3). The effect of spring applied N on the amount of soil mineralizable Nat the end of season for three rotations and years of on-farm experiment are shown in Table 1-3. It is apparent that fertilizer N rates did not significantly (p>.05) affect mineralizable nitrogen at the end of the season across rotations or years. Soil mineralizable N as estimated by the 7-day anaerobic incubation method was quite well correlated with field-measured N availability calculated by two different methods - N balance [equation 2] and by using the aboveground N uptake of check plots (Fig. 1-2). The highest correlation ( r2 = 0.78) for soil mineralizable N was observed when 29 40 30 bu oA 20 10 0 9/22/93 I-- Grass-wheat 12/22/93 Sampling date 2/02/94 Corn-wheat A-- Clover-wheat Fig. 1-1 Soil mineralizable N (closed symbols) and soil residual N (NO3-N and NH4-N (open symbols) taken throughout winter from three rotations in 1994 growing season. Table 1-2: Ammonium, nitrate and mineralizable nitrogen measured in top 30 cm of soil at spring fertilization in February during 1995-96 growing season Clover-wheat Rotation Treatment NO3-N NH4-N Min N Corn-wheat Rotation NO3-N NI-14-N Min N Grass-wheat Rotation NO3-N NH4-N Min N mg kg1 1Xt 2,5 4.3 16.0 2.0 4.2 17.5 2.7 3.9 33.6 2X 2.3 3.9 15.7 2.1 4.8 20.7 2.4 4.4 36.6 3X 2.6 4.6 18.3 2.8 6.9 17.9 2.4 3.5 33.5 4X 2.2 3.6 16.0 1.7 5.7 22.8 2.7 3.5 32.7 5X 2.5 4.0 16.6 2.2 5.7 23.0 2.6 4.2 38.8 Average 2.4 4.0 16.5 2.1 5.4 20.0 2.5 3.9 33.7 11 8 CV (%) 15 11 23 14 8 15 20 t Table values are averages across three plots assigned to a particular N rate treatment. Treatments were not yet applied when soil samples were taken hence data document field variation only. When analyzed as if plot groups represented treatments, there were no differences among treatments. Table 1-3: Mean values of mineralizable soil N measured in top 30 cm of soil at spring fertilization and after harvesting over three years and rotations. Corn-wheat Clover-wheat 1994 1995 1996 1994 1995 Grass-wheat 1996 1994 1995 1996 mg kg'' Pre-fertilization* 16.2 19.8 16.1 30.0 14.6 17.5 23.1 25.1 33.7 1Xt 14.8 29.3 13.4 34.2 19.6 15.6 18.7 27.4 24.8 2X 15.4 28.3 12.4 34.0 17.5 18.2 21.8 30.0 32.8 3X 14.4 32.4 12.1 37.5 23.6 19.7 19.1 17.0 23.6 4X 17.0 31.2 15.1 35.3 24.8 19.0 19.9 29.4 30.0 After harvesting 5X Average 12.7 15.4 30.1 13.1 19.3 35.3 21.4 18.4 41.1 19.9 25.9 30.3 CV 17.3 8.2 17.6 12.2 11.6 8.3 17.2 26.8 0.6 * Mean values of mineralizable N measured in top 30 cm of soil from check plots at spring fertilization Fertilizer N applied rates. Fertilizer N was applied at 56 kg N ha'. For grass-wheat rotation, during 1994 and 1995 growing seasons, N was applied at the increment of 67 kg N 32 N suppling capability was measured by the N balance method. This is likely because the N balance method takes into account N taken up by the plant and initial inorganic N (NH4-N and NO3-N) in the soil at the time of spring fertilization. The nitrogen balance method also estimates N mineralization over a short period of time - from fertilization to harvest ­ which makes it more comparable with the SMN test taken at fertilization. By considering these factors, better assessment of net N mineralization for the duration of the growing season can be expected by N balance method. Fiez et al. (1995) used a N balance method to calculate net nitrogen mineralization in a N use efficiency experiment and found satisfactory results. Broadbent and Carlton (1978) and Meisinger (1984) also suggested that determining the N uptake and an estimate of the change in soil mineral N of a field crop receiving no N fertilizer is the most satisfactory method of estimating the soil N supply in a given soil-crop-climate system. The soil supplied N (SSN) values obtained by the two different methods were plotted against soil mineral N test values to determine whether SSN could be expressed as a function of SMN test values (Fig. 1-2). To better examine the relationship between results obtained from anaerobic incubation method and SSN, we combined the individual data from small research plots, on-farm experiments across all years and the data obtained from mini plots conducted in the 1996 growing season. Soil supplied nitrogen increased as SMN test values increased. As shown in Fig. 1-2 (A and B), a linear relationship was found between SMN test and SSN calculated by the two methods The best linear correlation (R2 = 0.78) between SMN test values and SSN was obtained when the N budgeting method was used to estimate the N suppling capacity of the soil. However, total 33 10 15 (A) 20 25 Soil min N test (mg/kg) 30 Y = -46.2 + 5.42X SE = 0.4 RA2 = 0.78 10 (B) 0 On-farm Plots 15 20 25 Soil min N test (mg/kg) 30 0 Small research Plots v On-farm mini Plots Fig. 1-2: Relationship between mineralizable N test values and soil supplied N. Each value is an individual check plots from: on-farm experiment over three years, small research plots over two years, and on-farm mini plots over one year. Soil supplied N values were obtained by two methods (A) N uptake (B) N balance method 34 N uptake was also highly correlated with SMN (r2 = 0.69). Nitrogen uptake showed higher values typical of N uptake method. Mary and Recous (1994) argued that the problem in comparing a N index to only N uptake is that N uptake not only depends on N mineralization but also the amount of mineral N present in the rooting zone. Figure 1-2 shows more than a four fold increase in SSN, 20 to 110 kg N11-1, was observed when the SMN values increased from 14 to 29 mg N kg-1. While there is considerable variation in SSN values related to SMN values in the 17-25 mg N kg-1 range there is less variation in extreme values. This suggests that at a minimum, the SMN test can be used to predict low or high potential SSN values. It should also be noted that all three types of experiments exhibited a similar range of SMN and SSN values. To examine the effects of rotation, SSN data obtained from individual check plots from on-farm and small plot research experiments were plotted against SMN test values. Significant differences were found among rotations. As shown in Fig. 1-3, no consistent rotation effect was observed for wheat following clover or corn, but there was a pattern for wheat following grass and oat. Great variability for SMN test and SSN values was observed for wheat following both corn and clover while oat and grass rotations showed less variation (Fig. 1-3). Wheat following grasses had relatively high and uniform SMN test and SSN values across all years. The values ranged from 23 to 29 mg kg-i and 90 to 110 kg N ha-' for SMN test and SSN, respectively. Wheat following oats had the lowest SMN test and SSN values ranging from 11 to 17 mg kg1 and 12 to 39 kg N ha-1, respectively. 35 140 - A A :120 '&) 4 -'5-'Q., 0 100 o o 80 0 2 0A o 60 C4 .-_-, 0 o C 6? x 40 cA X 20 o X x 0 1°I 0 1 10 I 15 I I 1 I 25 20 Soil min N test (mg/kg) 0 Clover A Corn o Grass 1 1 30 X Oat Fig. 1-3: Relationship between mineralizable N test values and soil supplied N across four rotations. Each value is an individual check plots from: on-farm experiment over three years, small research plots over two years, and on-farm mini plots over one year. Soil supplied N values were obtained by using N balancing method. 36 Conclusions Soil mineralizable N estimated by the 7-day anaerobic incubation method correlated well with field-measured N availability. We concluded that the short-term anaerobic incubation method can satisfactorily predict the approximate soil N availability in western Oregon soils. This suggests that the results of a mineralizable N test could be used in making real-time N fertilizer recommendations for the region. Mineralizable N tests can be run on soil samples taken ahead of spring fertilization and results incorporated into N fertilizer recommendations for the following wheat crop. 37 References Aiman, N. 1992. Evaluation of the chemical methods for estimating potential mineralizable organic nitrogen in soils. M.S. Thesis. Kansas State Univ., Manhattan. Azam, F. 1990. Comparative effect of organic and inorganic nitrogen sources applied to a flooded soil on rice yield and availability. Plant and Soil. 25:255-262. Azam, F., F. W. Simmons, and R. L. Mulvaney. 1993. Mineralization of plant residues and its interaction with native soil N. Soil Biol. Biochem. 25:1787-1792. Azam, F., K. A. Malik, and F. Hussain. 1986. Microbial biomass and mineralization, immobilization of nitrogen in some agricultural soils. Bio. Fertil. Soils. 2:157-163 Beauchamp, E. G., W. D. Reynolds., D. Brasche-Villeneuve, and K. Kirby. 1986. Nitrogen mineralization kinetics with different soil pretreatment and management histories. Soil Sci. Soc. Am. J. 50:1478-1483. Binkley, D., and S. C. Hart. 1989. The components of nitrogen availability assessment in forest soils. Adv. Soil Sci. 10:57-112. Bock, B. R., and K. R. Kelly. 1992. Predicting N fertilizer needs for corn in humid regions. TVA Bull. Y-226. Natl. Fert. And Environ. Res. Ctr. Muscle Shoals, AL. Bremner, J. M. 1965. Nitrogen availability indexes. In C.A. Black et al. (ed.) Methods of soil analysis, part 2. Agronomy. 9:1149-1176. Broadbent, F. E., and A.B. Carlton. 1978. Field trials with isotopically labeled nitrogen fertilizer. p. 1-14. In D. R. Nielsen and J. G MacDonald (ed.) Nitrogen in environment. Vol. 1. Nitrogen behavior in field soil. Academic Press, New York. Bundy, L. G., and E. S. Malone. 1988. Effect of residual profile nitrate on corn response to applied nitrogen. Soil Sci. Soc. Am. J. 52:1377-1383. Cabrera, M. L., and D. E. Kissel. 1988. Evaluation of a method to predict nitrogen mineralized from soil organic matter under field conditions. Soil Sci. Soc. Am. J. 52: 1027-1031. Campbell, C. A., Y. W. Jame, and R. D. Jong. 1988. Predicting net nitrogen mineralization over a growing season: Model verification. Can. Soil Sci. 68:537­ 552. 38 Collins, H. P., P. E. Rasumussen, and C.L. Douglas, Jr. 1992. Crop rotation and residue management effects on soil carbon and microbial dynamics. Soil Sci. Soc. Am. J. 56:783-788. Copeland, P. J., and R. K. Crookston. 1992. Crop sequence affects nutrient composition of corn and soybean growth under high fertility. Agron. J. 84:503-509. Cox, F. 1985. Survey of state soil testing laboratories in the united States. Soil Sci. Soc. Am. Madison, WI. Dahnke, W. C., and G. V. Johnson. 1990. Testing soils for available nitrogen. p. 127-139. In R. L. Westerman (ed.) Soil testing and plant analysis. ASA, CSSA, and SSSA, Madison. WI Ellert, B. H., and J. R. Bettany. 1992. Temperature dependence of net nitrogen and sulfur mineralization. Soil Sci. Soc. Am. J. 65:1133-1141. Eno, C. F. 1960. Nitrogen production in the field by incubating the soil in polyethylene bags. Soil Sci. Soc. Am. Proc. 47:277-279. Fiez, T. E., W. L. Pan., and B. C. Miller. 1995. Nitrogen use efficiency of winter wheat among landscape positions. Soil Sci. Am. J. 59: 1666-1671. Forth, D. H. and B. G. Ellis. 1988. The soil nitrogen cycle. In Soil Fertility. John Wiley & Sons. Ins. p. 69-73. Fox, R. H. and W. P. Piekielek. 1984. Relationship between anaerobically mineralized nitrogen, chemical indexes, and nitrogen availability to corn. Soil Sci. Soc. Soc. Am. J. 48:1087-1090. Fox, R. H. and W. P. Piekielek. 1978. Field testing of several nitrogen availability indices. Soil Sci. Am. J. 42:747-750. Franzluebbers, A. J., F. M. Hons., and D. A. Zuberer. 1994. Long-term changes in soil carbon and nitrogen pools in wheat management systems. Soil Sci. Soc. Am. J. 58:1639-1645 Gelderman, R. H., W. C. Dahnke, and L. Swenson. 1988. Correlation of several N indices for wheat. Commun. Soil Sci. Plant Anal. 19 (6):755-772. Gianello, C., and J. M. Bremner. 1986. Comparison of chemical methods of assessing potentially available organic nitrogen in soil. Commun. Soil Sci. Plant Ana. 17:215-236. 39 Harper, J. E. 1984. Uptake of Organic Nitrogen Forms by Roots and Leaves. In R. D. Hauck (ed.) Nitrogen In Crop Production. Am. Soc. Agron. Madison, WI. Hassink, J. 1994. Active organic fractions and microbial biomass as predictors of N mineralization. Eur. J. Agron. 3:257-26. Houot, S., J. A. E. Molina, R. Chaussod, and C.E. Clapp. 1989. Simulation by NCSSOIL of net mineralization in soil from the Deherain and 36 Parcelles fields at Grignon. Soil Sci. Soc. Am. J. 53:451-455. Jarvis, S. C., A. S Elizabeth., M. A. Shepherd, and D. S. Powlson,. 1996. Nitrogen mineralization in temperate agriculture soils: Processes and measurement. Advances in Agronomy. Vol. 57. Academic Press. pp 187-230. Keeney, D. R. 1982. Nitrogen availability indices. p 711-733. In A.L. Page et al. (Ed.) Methods of Soil Analysis. Part 2. Agron. Monrog. ASA and SSSA, Madison, WI. Keeney, D. R., and D. W. Nelson. 1982. Nitrogen inorganic forms. p. 643-698. In A. L. Page et al. (ed.) Methods of soil analysis. Part 2. 2nd ed. Agron. Monogr. 9. ASA ans SSSA, Madison, WI. Liang, B. C., M. Remillard, and A. F. MacKenzie. 1991. Influence of fertilizer, irrigation, and non-growing season precipitation on soil nitrate-nitrogen under corn. J. Environ. Qual. 20:123-128. Linn, D. M., and J. W. Doran. 1984. Effect of water-filled pore and space on CO2 and NO2 production in tilled and no-tilled soils. Soil Sci. Soc. Am. J. 48:1267-1272. Loiseau, P., R. Chaussod, and R. Delpy. 1994. Soil microbial biomass and in situ nitrogen mineralization after 20 years of different nitrogen fertilization and forage cropping system. Eur. J. Agron. 3:327-332. Magdoff, F. R. 1991. Understanding the Magdoff pre-side dress nitrate test for corn, Prod. Agric. 4:297-305. Maples, R., J. G. Keogh, and W. E Sabbe. 1977. Nitrate monitoring in cotton production in Loring-Calloway silt loam. Arkansas Agric. Exp. Stn. Bull. No. 825. Marumoto, T., J. P. Anderson, and K. H. Domsch. 1982. Mineralization of nutrients from soil microbial biomass. Soil Bio. Biochem. 14: 469-475. 40 Mary, B., and S. Recous. 1994. Measurement of nitrogen mineralization and immobilization fluxes in soil as a mean of predicting net mineralization. Eur. J. Agron. 3:291-300. Meisinger, J. J., R. F. Mulford, and J. S. Stangarone. 1987. Effect of residual N on winter wheat in Maryland. p. 209. In Agronomy Abstracts. ASA, Madison, McKenney, D. J., S. W. Wang., C. F. Drury, and W. I. Findlay. 1995. Denitrification, immobilization, and mineralization in nitrate limited and non limited residueamended soil. Soil Sci. Soc. Am. J. 59:118-124. McTaggert, I. P. and K. A. Smith. 1993. Estimation of potentially mineralizable nitrogen in soils by KC1 extraction: Comparison with plant uptake in the field. Plant Soil. 157-184. Michrina, B. P., R. H. Fox, and W. P. Piekielek. 1981. A comparison of laboratory, greenhouse and indicates of nitrogen availability. Commun. Soil Sci. Plant Anal. 12:519-535. Neve, S. D., J. Pannier, and G. Hofman. 1994. Fraction of vegetable crop residues in relation to in situ N mineralization. Eur. J. Agron. 3:267-272. Ower, J. C. 1980. Nitrate leaching as affected by artificial drains. M.S. Thesis Oregon State University, Corvallis, OR Palm, C. A., and P. A. Sanchez. 1991. Nitrogen release from some tropical legumes as affected by lignin contents. Soil Biol. Biochem. 23:83-88. Power, J. F., and J. W. Doran. 1984. Nitrogen Use in Organic Farming. In R. D. Hauck (ed.) Nitrogen In Crop Production. Am. Soc. Agron. Madison, WI. Qian. P., J. J. Schoenau, and A. Braul. 1993. Assessing mineralizable soil organic N and S using anion exchange. p. 257, in Agronomy Abstracts. ASA, Madison, Wl. Rauschkolb, R. S., T. L. Jackson, and A. I. Dow. 1984. Management of nitrogen in the pacific states. p. 765-777. In R.D. Hauck (ed.) Nitrogen in Crop Production. ASA. Madison, WI. Recous, S., J. M. Machet, and, B. Mary. 1988. The fate of 15 N urea and ammonium nitrate applied to a winter wheat crop. Plant Soil. 112:215-224. Rees, R. M., I. P. McTaggart., K. A. Smith. And E. A. Stockdale. 1994. Methodology for the study of N mineralization in the field. Eur. J. Agron. 3: 301-309. 41 Rice, C. W., and J. L. Hav lin. 1994. Integrating mineralizable nitrogen indices into fertilizer nitrogen recommendations. p. 1-13. In Soil testing: Prospects for improving nutrient recommendations. SSA Special publication number 40. Madison WI. Roth, G. W. 1987. Evaluation of plant tissue and soil test for predicating nitrogen fertilizer requirements of winter wheat. P.d. Thesis. Pennsylvania State Univ. University Park, PA. Roth, G. W., and R. H. Fox. 1990. Soil nitrate accumulations following nitrogen-fertilized corn in Pennsylvania. J. Environ. Qual. 19:243-248. Sarrantonio, M., and T. W. Scott. 1988. Tillage effects on nitrogen availability to corn following a winter green manure crop. Soil Sci. Soc. Am. J. 52:1661-1668. Sebastain, K. D. 1995. Nitrogen fertilization of winter wheat grown in four rotations in western region. M.S. Thesis. 101. pp. Oregon State University, Corvallis, OR. Serna, M. D., and F. Pomares. 1992. Evaluation of chemical indices of soil organic nitrogen availability in calcareous soil. Soil Sci. Soc. Am. J. 65:1486-11491. Smith, J. L. 1994. Cycling of nitrogen through microbial activity. In advances in soil Science. Lewis publishers. Pp 91-119. Smith J. L., R. I. Papendick., D. F. Besdicek, and L. M. Lynch. 1993. Soil organic matter dynamics and crop residue management. In Soil applications in agricultural and environmental management. (F.B. Metting Jr., Ed), pp. 65-94. Microbial Ecology. Marcel Dekker Inc, New York. Sparling, G. P. and, D. J. Ross. 1988. Microbial contributions to the increased nitrogen mineralization after air-drying of soils. Plant Soil. 105:163-167 Stanford, G. 1982. Assessment of soil N availability. p. 651-688. In F. J. Stevenson (ed.) Nitrogen in Agricultural Soils. Agron. Monogr. 22. ASA, CSSA, and SSSA, Madison, WI. Stanford, G., and S. J. Smith. 1972. Nitrogen mineralization potentials of soils. Soil Sci. Soc. Amer. Proc. 36:465-472. Thicke, F. E., M. P., 0. B. Hesterman, and C. C. Sheaffer. 1993. Soil nitrogen mineralization indexes and corn response in crop rotations. Soil Science. 156:322-334 42 Wang, J. G., and R. L. Bakken. 1989. Nitrogen mineralization in the rhizosphere and non-rhizoshere soil, effect of spatial distribution of N-rich and N-poor plant residue. In: J.A. Hansen. and K. Henriksen (eds.) Nitrogen in organic wastes applied to soils. London: Academic Press, pp 81-97. Wani, S. P., W. B. McGill, and L. A. Robertson. 1991. Soil N dynamics and N yield of barley grown on Breton loam using biological fixation or fertilizer. Bio. Fert. Soils. 12:10-18. Westfall, D. G. 1984. Management of nitrogen in the Mountain states. p. 751-763./n R.D. Hauck (ed.) Nitrogen in crop production. ASA. Madison, WI Whitmore, A. P., and J. R Groot. 1994. The mineralization of N from finely or coarsely chopped crop residues: measurement and modeling. Eur. J. Agron. 3:367-373. Wilson, C. E., R. J. Norman, and B.R. Wells. 1994. Chemical estimation of nitrogen mineralization in paddy rice soils: I. Comparison to laboratory indices. Commun. Soil Sci. Plat Anal. 25:573-590. 43 CHAPTER 3 A MODEL FOR MAKING FIELD-BASED NITROGEN RECOMMENDATIONS FOR WINTER WHEAT IN WESTERN OREGON Abstract Early determination of crop N status allows for greater options in N fertilizer management. Incorporating information about crop N requirements, soil testing, and plant analysis at early growth stages will be very helpful towards optimizing the fertilizer N recommendations for winter wheat. A model based on early spring soil and tissue analysis was developed and evaluated for predicting the need for additional N fertilizer on winter wheat. On-farm N response trials were established over three years to develop the model. Two field-scale validation trials were run in another year. On-farm trials were conducted in growers' fields at three different location across the Willamette valley of western Oregon during the 1994­ 1996 growing seasons. Each site of these experiments represented a crop rotation system that is commonly used in the valley. Rotations were soft white winter wheat following grass seed, sweet corn or a legume. Four treatments, including a check receiving no nitrogen, were used at each site during the 1993-94 and 1994-95 growing seasons. In 1996-97, two trials were conducted to further check the validity of the proposed model. Rotations were soft white winter wheat following grass seed or sweet corn. Three treatments were used at each site. At the site where wheat followed corn, the predicted 44 optimum N rate was 168 kg N ha-1. A low (84 kg N ha-1) and intermediate (140 kg N ha-1) rate were selected as other treatments in this trial. At the site where wheat followed grass seed, N was applied at the rate of 84, 112, and 140 kg N ha-1. The 112 kg N ha-1 rate was the optimum rate predicted from our model. The 84 kg N ha-1 and 140 kg N ha.-1 rates were selected to bracket the recommended rate (± 28 kg N ha-1). Response of winter wheat to fertilizer N varied greatly among rotations and years in this study. Wheat following corn was highly responsive to added fertilizer N while wheat following grass seed was less responsive. Wheat following grass seed had high soil supplied N which depressed the yield even at moderate fertilizer N rates. This finding contradicts conventional wisdom suggesting that higher N rates are needed following grass. This study documents a difference between perennial grasses and cereal grain crops in rotation. The average yield with no fertilizer for wheat following corn and wheat following legumes were about the same. Optimum N rates predicted by our proposed model were closely related to the N rates required to obtain maximum economic yield. This study overall shows that the model appears to accurately assess field-specific optimum fertilizer N status and that it can be used to make fertilizer N recommendations for the region. 45 Introduction The assessment of optimum N fertilizer rates for winter wheat (Triticum aestivum L.) is important for economic and environmental sustainability. Nitrogen alters plant growth more than any other mineral nutrient. In wheat a suboptimal supply of N can dramatically reduce dry matter and subsequently grain yield (Power and Allessi, 1978; Robinson et al., 1979; Donohue and Brann, 1984; Nielsen and Halvorson, 1991), while oversupply of N may cause lodging, disease and lower grain quality (Boquest and Johnson, 1987; Beuerlein et al., 1992; Memon and Jamro, 1988). The determination of optimum N fertilizer rates for winter wheat is a major unsolved problem in most humid region of the world (Stanford, 1982). Determining precise N fertilizer rates requires the consideration of yield goals, N requirement of the crop, soil N tests, and plant analysis. The amount of fertilizer N necessary will vary depending on yield desired, residual N and the N supplying capacity of the soil. Fertilizer N recommendations are mostly influenced by yield goals. Nearly 80 % of wheat growers in the great plains overestimate their yield goals and apply excess N fertilizer (Goos and Prunty, 1990; Schepers et al., 1986). Realistic yield goals for each field are crucial in determining optimum fertilizer N rates. An estimate of the fertilizer N needs of a particular crop cannot be determined quantitatively without knowledge of the crop's requirement for this element. Crop N requirement is a function of yield potential and the amount of N required per unit of yield. Stanford and Legg (1984) defined the N requirement as the minimum amount of N in the 46 above ground portion of crops associated with maximum production. Nitrogen rate experiments are the most effective way of determining N requirements for a crop. Yield will continue to increase as N is supplemented when N is a limiting factor in plant growth. There is essentially no further increase in yield with increasing N content once the crop requirement for N is met. Soil mineral N (NI-14-N + NO3-N) testing before spring fertilization has proven a useful tool for adjusting N fertilizer requirements in the semi-arid and semi-humid regions of the U.S. (Maples et al., 1977; Westfall, 1984; Rauschkolb et al., 1984; Cox, 1985). Despite complications such as mobility, leaching, and nitrification, soil tests for residual NO3-N have been found to be useful in determining N fertilizer needs of crops (Gelderman et al., 1988). A soil nitrate test before planting appears to be well related to the fertilizer requirements of spring wheat in the Northern Plains (Fox and Piekielek, 1978). In a study conducted on the Eastern Shore of Maryland, Meisinger et al. (1987) found that the N requirement of a wheat crop could be effectively adjusted by measuring residual spring N status of the soil. Soil tests for residual N can improve prediction of crop N fertilizer requirements in humid regions of U.S., but their adaptation in fertilizer recommendation depends on the frequency of significant profile NO3-N carryover (Vanotti and Bundy, 1994). In the humid Midwest, measurements of NO3-N in the soil profile before corn (Zea mays L.) planting effectively predicted N response and the amount of supplemental N needed for profitable production (Schmitt et al., 1991; Bundy et al., 1992). Usefulness of a soil N test in a humid region was also reported by Vanotti and Bundy (1994). Their results illustrated that 47 large variations, 32 to 106 kg N ha-1, in N carryover made soil testing for NO3-N an important tool to optimize N fertilizer use in the humid region of Wisconsin. Wehrmann and Scharpf (1979) found a strong correlation between optimum fertilizer rates and soil mineral N. They developed a predictive system (the Nmin method) from this relationship which not only predicts the optimum fertilizer N rate but also identifies fields not needing fertilization. Many researchers used their approach to optimize fertilizer N for winter wheat (Baethgen and Alley, 1989; Bundy et al., 1992). Soil supplied N is derived either from residual inorganic N or from organic N that becomes converted to inorganic N by soil microbes. Researchers have long been aware that a small percentage of organic N is mineralized in the course of the growing season and can significantly contribute to nitrogen requirements of the crop (Foth and Ellis,1988; Harper, 1984; Power and Doran, 1984; Marumoto et al.,1982). Biological immobilization of soil inorganic N occurs simultaneously with mineralization. A significant portion of mineralized N is immediately assimilated by soil microbial biomass and transformed into organic cell constituents (Mary and Recous, 1994). During mineralization-immobilization, soil microbial biomass temporarily serves as source and sink of easily mineralizable nitrogen (Smith, 1994). Soil microbial biomass predominantly assimilates NH4-N during the immobilization process, but in the absence of NH4-N, can use NO3 -N as an energy source (Recous et al., 1988; Azam et al., 1986). The difference between gross rates of mineralization and immobilization is net mineralization. Net mineralization is strongly dependent on C:N ratio of the organic matter (Whitmore and Groot, 1994; Neve et at., 1994). The amount of N returned to the soil by crop residues depends on the quality and 48 quantity of the substrate. Crops grown in rotation often produce more and higher quality plant dry matter than those grown in monoculture (Copeland and Crookston, 1992; Collins et al., 1992). Change in crop sequence changes soil temperature, soil moisture, crop rooting and residue inputs. Fertilizer N requirement for optimum grain yield is often reduced in rotation compared to monoculture (Franzluebbers et al., 1994). Cereal crops grown after a legume often have a higher yield as compared to those grown after a non-legume crop (Wani et al., 1991). This is because legume residues generally have a low C:N ratio and large fraction of readily available N. Breland (1994) studied the decomposition dynamics of white clover in a controlled environment. Net mineralization was estimated as the difference between that in amended and unamended soil, respectively. He found that 38-56 percent of the nitrogen in clover shoot dry matter was mineralized during the initial period (52 days) of rapid mineralization. Thicke et al. (1993) found that the decomposition of incorporated legume residues can have significant, but extremely variable, impact on total N mineralization. McKenney et al. (1995) concluded that legume residues can provide a definite advantage compared with grass and corn residue in term of N conservation depending on the aeration status of the soil. Legume residues not only provide a great source of readily available N to other crops (Azam, 1990), but can also increase long-term soil fertility (Palm and Sanchez, 1991). This increase is mainly because of the conversion of a portion of biologically-fixed N into the stable humus form (Azam et al., 1993). In addition to the enhancement of N fertility, legumes also affect soil properties such as water holding capacity, cation exchange capacity, buffer capacity, soil porosity and 49 infiltration (Burnett, 1975; Cook, 1988; Fyson and Oaks, 1990). In western Oregon, a variety of crop rotations create large differences in soil N supplying capacity which are not reflected in soil tests for inorganic N. In a study carried out in western Oregon, Sebastian (1995) measured N recovery by unfertilized winter wheat ranging from 96 to 192 kg ha 1. Assessment of mineralizable N at the beginning of the growing season and coupling the results with soil mineral and plant analysis could be helpful in improving fertilizer N recommendations in the region. Plant tissue analysis at early growth stages are another important method of estimating optimum fertilizer N rates and have successfully been used as an indicator of N fertilizer requirements for wheat by many researchers (Engel and Zubriski, 1982; Donohue and Brann, 1984; Becker and Authammer, 1982). In regions were soil testing for N has been impractical due to heavy precipitation, plant tissue testing could be a promising method of estimating fertilizer N requirements for winter wheat (Fox and Piekielek, 1984). Many fertilizer N recommendations are based on the analysis of plant tissue samples taken at Feekes 5 (Large, 1954) in the spring. Vaughan et al. (1990) found that late tillering (Feekes 5) is the most appropriate and usable growth stage for plant sampling in order to make tissue-based N recommendations. They also suggested that total N should be calculated from whole plant samples. Roth et al. (1989) reported that whole plant N concentration at Feekes 5 rather than N uptake is the most accurate method of predicting N deficiency. Scharf (1993) found tissue N content a useful tool in adjusting optimum N rate in Virginia. 50 A method for making field-specific N rate recommendations for winter wheat at early growth stages was reported by Baethgen and Alley (1989). Their method used crop N uptake or plant N concentration at Feekes 5 as the basis of the N rate recommendations. Strong correlation was found between uptake or plant N concentration at Feekes 5 and optimum N rates. Similar results were reported by Batey (1977). He also found a high correlation between crop uptake in spring and the rate of fertilizer N required to obtain maximum grain yield. Peter et al. (1993) used this approach and also found a good relationship between tissue N and optimum fertilizer N rates. Economic analysis indicated that tissue-based N rate recommendation increased profit by an average of $36 hat relative to traditional N applications. Application of fertilizer N is often necessary to supplement soil supplied N in commercial cropping systems. Efficient N management utilizes soil N as much as possible, adding fertilizer N to enhance crop growth only when necessary (Sebastian, 1995). The amount of fertilizer N required will vary depending on yield desired, residual N and the N suppling capacity of the soil. Incorporating information about crop N requirements, soil testing, and plant analysis at early growth stages will be very helpful towards optimizing the fertilizer N recommendations for winter wheat. The objective of this study was to evaluate the suitability of a N balance model for predicting the need for additional N fertilizer on winter wheat under western Oregon conditions. 51 Materials and Methods Nitrogen Balance Model A model similar to that proposed by Rice and Hav lin (1994) was developed to calculate optimum fertilizer N rates. Recommended nitrogen rates were determined by this model: Recommended N = 300 - (Nr + Nmin N p) [1] Where, N,. = Residual inorganic N (NH4 -N + NO3-N) kg ha' at Feekes 5 Nrith, = Soil mineralizable N kg ha' at Feekes 5 1\cp = Plant N uptake kg ha' at Feekes 5 The 300 kg N ha.-1 is the total N (residual N + mineralizable N + fertilizer N) estimated to be required for maximum yield in Willamette Valley of western Oregon. Analysis of nitrogen response data for soft white winter wheat by Neil Christensen and John Hart, Soil Scientists, Department of Crop and Soil Science, Oregon State University, suggested that this total quantity of N maximized yield over an array of environmental and production conditions. Data from over twenty years and dozens of trials were analyzed. Total N requirement was estimated by adding the amount of N recovered in unfertilized plots (an estimate of mineralized N) to the amount of applied fertilizer N that gave maximum yield in each trial. The average total N requirement was about 300 kg N ha' (unpublished data) and showed little variation across an approximately 2X range in grain yield. This uniformity in N needed to maximize yield under an array of environmental conditions 52 suggested that it may be possible to model N need. Nitrogen response trials were established in three years to develop model and two validation field-scale trials were run in one year. Each type of trial is discussed below. On-farm Trials On-farm trials were conducted in growers' fields at three different locations across the Willamette Valley of western Oregon during the 1994-1996 growing seasons. The 1993-94 experiment was part of fellow graduate student Kevin Sebastian's (1995) MS Thesis. Each site of these experiments represented a crop rotation system that is commonly used in the Valley. Rotations were soft white winter wheat following grass seed, sweet corn or a legume. The experimental design was a randomized complete block with three replications on each site. Plot size varied site-to-site depending on size of machinery used by the grower. An average plot size of 90m X 10m was used. Nitrogen fertilizer was applied as urea (46-0-0) at approximately Feekes 5 in one application with drop or spinner spreader. Four treatments, including a check receiving no nitrogen, were used at each site during the 1993-94 and 1994-95 growing seasons. At corn and legume rotation sites, N was applied at the rate of 0, 56, 112 or 168 kg N ha-1. Since less residual nitrogen was expected following grass in rotation, slightly higher rates- 0, 67, 134, or 201 kg N ha'- were used in the first two years. During the 1995-96 growing season, five N rates - 0, 56, 112, 168 or 224 kg N ha-1- were used in all three rotations. The location, soil series, cropping history and wheat varieties used in the experiments are listed in Table 2-1. 53 Table 2-1: Location, previous crop, variety and soil series for experimental sites. Grower County/City Previous crop Wheat variety 1994 Jones Polk/Amity Clover Madsen Amity Fine-silty, mixed mesic, Argiaquic Xeric Argialbolls Volker Benton/Monroe Corn Gene Malabon Fine, mixed, mesic Pachic Ultic Argixerolls Van Leeuwen Linn/Halsey Tall Fescue Gene Malabon Fine, mixed, mesic Pachic Ultic Argixerolls 1995 Ruddenklau Polk/Amity Clover Gene Amity Fine-silty, mixed mesic, Argiaquic Xeric Argialbolls Volker Benton/Monroe Corn Gene Chehallis Fine-silty, mixed, mesic Cumulic Ultic Haploxerolls Van Leeuwen Linn/Halsey Tall Fescue Stephens Woodburn Fine-silty, mixed, mesic Aquultic Argixerolls 1996 Ruddenklau Polk/Amity Clover Gene Amity Fine-silty, mixed mesic, Argiaquic Xeric Argialbolls Volker Benton/Monroe Corn Gene Malabon Fine, mixed, mesic Pachic Ultic Argixerolls Chipman Benton/Albuny Perennial Raygrass Madsen Willamette Fine-silty, mixed mesic Ultic Haploxeralfs 1997 Mulkey Polk/Monmouth Annual Raygrass Madsen Willamette Fine-silty, mixed mesic Ultic Haploxeralfs Jones Benton/Corvallis Corn Stephens Chehallis Fine-silty, mixed, mesic Cumulic Ultic Haploxerolls Soil Series 54 Soil samples were collected at approximately one month intervals from each site during the 1993-94 and 1994-95 growing seasons. Samples were taken at four depths- 0­ 30, 30-60, 60-90 and 90-120 cm. A single composite sample representing the entire plot area was used prior to spring fertilization. Approximately ten cores were taken to make this composite sample. Analysis of soil data for NH4-N and NO3-N from these two growing seasons indicated that sample from 0-30 cm depth taken at Feekes 5 was adequate to assess soil N status (data not shown). During the 1995-96 growing season, pre-fertilization soil samples were collected from individual plots to a depth of 30 cm. Each sample was a composite of approximately 10 cores. Intensive soil sampling was performed just after harvesting at each site on each plot in all years to three depths- 0-30, 30-60, and 60-90 cm. To calculate total N uptake, plant tissue samples were taken at Feekes GS 5 ( prior to fertilizer application) and at maturity. At Feekes 5, ten representative samples of 30 cm of row were collected from each plot by cutting the plants at soil level. Whole plant samples were weighed, ground and analyzed for nitrogen content. At maturity, nine one- meter row sections were cut at soil level from each plot to determine dry matter. Heads from plants were removed and threshed separately. Representative subsampling was performed for each sample. Subsamples of grain, straw and chaff were analyzed for nitrogen content. Plant N uptake (kg N ha') was calculated by multiplying the N concentration in the tissue by dry matter production (kg DM ha-1). Plant height and lodging measurements were taken prior to harvest. Grain yield was obtained from individual plots. Growers used their equipment to harvest the plots. Grain yield was 55 determined on site through use of a weigh wagon accurate to ± 1 kg. A grain sample was saved for test weight, protein and 1000 kernel weight determination. Small Plot Research Station Trials Fertility trials were established during the 1993-94 growing season at the Hyslop Experimental Station of the Department of Crop and Soil Science, Oregon State University, Corvallis, Oregon. Work continues on these trials. The experiment was arranged as a spilt plot design with four replications having rotation (clover or oat) as main plots and fertilizer treatments as sub-plots. Rotation treatment (main plots) size was 12m X 45m, while fertilizer treatment (sub plots) size was 12m X 9m. Five fertilizer treatments - 0, 50, 100,150 or 200 kg N ha.-1 - were used. Rotations include winter wheat following clover and winter wheat following oat. The site had been fallow in 1993. Crimson clover was planted on all plots in fall 1994. In spring 1995, two plots per replication were fallowed, one was planted to oat and the fourth was left in clover. Clover and oat were allowed to mature, were harvested and crop residue was incorporated. Wheat was planted on the clover and oat stubble plots in fall 1995 and crimson clover on one of the fallowed plots to establish the next rotation cycle. Oats were planted in spring 1996. Data were collected for 1995-96 and 1996-97 growing seasons. Soil samples were collected pre-plant and post-harvest. An intensive pre-plant sampling was done in fall 1995 by taking soil at 30 cm increments to a depth of 150 cm from individual plots. This was done to assess the residual nitrogen status of the experimental site. Post-harvest sampling was performed on 0, 150 and 200 kg N ha-1 56 treatment plots within each rotation. Samples were analyzed for ammonium and nitrate concentrations. Plant tissue samples were taken at Feekes 5 and at maturity for dry mater yield and nitrogen uptake. Four above ground plant samples of 1.5 meter of row were cut from drill strips in each sub-plot. Heads were cut, threshed and weighed. Grain and straw were analyzed separately for their nitrogen content by combustion analyzer. Plant N uptake (kg N ha') was calculated by multiplying the N concentration in the tissue by day matter production (kg DM ha'). Plant height and lodging were measured prior to harvest. Plots were harvested with a small plot combine harvester. Harvested grain was cleaned and analyzed for yield, test weight, protein content by whole grain NIR analyzer and 1000 kernel weight. Model Validation Trial In 1996-97, two trials were conducted to further check the validity of the proposed model. Trials were conducted in growers fields at two location in the Willamette Valley. Rotations were soft white winter wheat following grass seed and sweet corn. The experimental design was a randomized complete block with three replications at each site. Plot size varied depending on size of machinery used by the grower. An average 90mX10m plot size was used. Nitrogen fertilizer was applied as urea (46-0-0) at approximately Feekes 5. Three treatments were used at each site. We were not interested in N response per se but bracketing N rates were used at each site. At the wheat following corn rotation site, the predicted optimum N rate was 168 kg N ha'. Previous corn rotation 57 trials had not shown an economic yield respond beyond this rate hence a low (84 kg N ha-1) and intermediate (140 kg N ha-1) rate were selected as other treatments in this trial. The cooperating grower was interested in lower N rates. Spreader calibration error resulted in an intermediate rate of 132 kg N ha-1 At the wheat following grass seed rotation site, N was applied at the rate of 84, 112, and 140 kg N ha-1. The 112 kg N ha-1 rate was the recommended rate from our model. The 84 kg N ha-1 and 140 kg N ha-1 rate were selected to bracket the recommended rate (± 28 kg N ha-1). The location, soil series, cropping history and wheat varieties used in the experiments are listed in Table 2-1. Soil samples were collected before fertilizer application and after harvest. At prefertilization, soil samples were taken from a 0-30 cm depth while after-harvest samples were taken at three depths- 0-30, 30-60, and 60-90 cm from individual plots. Each sample was a composite of approximately 10 cores. Plant tissue samples were taken at Feekes GS 5 ( prior to fertilizer application) and at maturity to calculate total N uptake. At Feekes 5, ten representative samples of 30 cm of row were collected from each plot by cutting the plants at soil level. Whole plant samples were weighed, ground and analyzed for nitrogen content. At maturity, nine one- meter row sections were cut at soil level from each plot to determine dry matter. Heads from plants were removed and threshed separately. Representative subsampling was performed for each sample. Subsamples of grain, straw and chaff were analyzed for nitrogen content. Nitrogen uptake (kg N ha-1) was calculated from day matter production (kg DM ha-1) and corresponding plant N concentration. 58 Maximum Economic Yield Equations The validity of the N balance model was checked by comparing the recommended N rates determined by the model with N rates determined in 1996-97 trials to obtain maximum economic yield. To calculate the fertilizer rate required for maximum economic yield, regression equations were developed to describe wheat grain yield response to N fertilizer applied at Feekes 5 (Baethgen and Alley, 1989). The relationship between grain yield and fertilizer was quadratic in shape for each rotation (Mason, 1987). The quadratic equation used is as follow: Y = a + biN - b2N2 [2] Where, Y = Grain yield (kg ha-1) a = Intercept b1 = Linear regression coefficient b2 = Quadratic regression coefficient N = Fertilizer N rate (kg ha-1) The maximum economic N rate for any given response curve is the N fertilizer rate value that makes the first derivative of the response function equal to the price ratio of N fertilizer to wheat ($kg N to $ kg wheat)( Heady et al. 1955). The fertilizer rate for maximum economic response was calculated as follows: Y = a + biN - b2N2 Setting the first derivative equal to N price/wheat price [2] 59 dY/dN = bi - 2 b2 N = PN/Py = r r = bi - 2 b2N 2 b2N = bl - r N = (bi - r)/ 2b [3] Where, r = price ratio of N fertilizer to wheat ($ 0.60 per kg N to $ 0.14 and 0.16 per kg wheat) b1 and b2 are regression coefficients. Soil Analysis Soil and plant tissue samples were analyzed in the Central Analytical Lab (CAL) at Oregon State University, Corvallis, Oregon. Plant samples were dried at 70 °C in a forced air oven, weighed and ground in a Wiley mill to pass a 1 mm mesh screen. The N content of wheat grain and straw was determined by a Leco CNS 2000 (Leco corporation, St. Joseph, MI) combustion analyzer. Soil inorganic N (NH4-N and NO3-N) was determined using the modified KCI extraction method described by Keeney and Nelson (1982). Twenty-gram soil samples were placed in 250 mL bottles and 75 mL of 2 N KC1 extracting solution was added. Vessels were shaken on a mechanical shaker for one hour. The extraction solution was filtered through Whatman No. 42 filter paper. The NH4-N and NO3-N content of the extract was determined with an ALPKEM rapid flow analyzer (RF-300) which complexes NH4-N with salicylate to form indophenol blue. This color was intensified with sodium nitroprusside and measured at a wavelength of 660 nm. The NO3-N concentration were 60 determined with the same equipment used for NH4-N analysis by reducing nitrate via a cadmium reactor and complexing the NO3-N with sulfanilamide and N-(1-Napthyl)­ ethylenediamine dihydorchloride to form a red-purple color. The color intensity was measured at a wavelength of 540 nm. Anaerobic Incubation Soil mineralizable N was determined using a short-term anaerobic incubation method described by Keeney (1982) slightly modified by increasing the sample size. Through use of a sample splitter, a 20 g soil sample was obtained and placed in a 250 mL extraction bottle. Fifty mL of distilled water was added to each bottle so that the soil became completely saturated. Bottles were made air tight by putting a plastic cover under their lids. Samples were placed in an incubator for 7 days (168 h) at 40 °C plus or minus 0.5 °C. After incubation, samples were carefully removed from the incubator and 50.0 mL of KCl was added. Vessels were shaken on a mechanical shaker for one hour. The extracting solution was filtered through Whatman No. 42 filter paper. Final NH4-N content of the extract solution was determined from incubated samples. Initial NI14-N values were subtracted from the final values to obtain the amount of N mineralized. 61 Results and Discussion Optimum fertilizer N rates for winter wheat were estimated by the following N balance model. Recommended N = 300 - (N, + Nuun + N 1,) [1] Where N, and Nmu, are the soil inorganic N (NH4 -N + NO3-N) and soil mineralizable N kg ha-' at Feekes 5 before fertilizer application, respectively, and Nup is the total N (kg ha-1) taken up by the crop at Feekes 5 prior to fertilization. The 300 N kg ha-1 is the total N (residual N + mineralizable N + fertilizer N) assumed to be needed for maximum yield in Willamette Valley of western Oregon. The parameters used in the model were determined at the time of spring fertilization in order to assess optimum N rates. Parameter values and predicted fertilizer N need for the various trials conducted as part of this study are shown in Table 2-2. At on-farm sites, the amount of soil inorganic N in the top 30 cm of soil at the time of spring fertilizer application was low and similar through all rotations over years. The average inorganic N across all rotations over years was only 27 kg N ha.-1 (Table 2-2). This is likely because of leaching and/or denitrification losses due to heavy precipitation during winter months (Sebastian, 1995). Soil mineralizable N in the top 30 cm of soil varied greatly among rotation over years. Amount measured prior to spring fertilization ranged from 53 kg N ha"' in wheat following corn in 1995 to 116 kg N ha-' in the wheat following grass seed in 1996. In 1995 and 1996, wheat following grass had relatively high soil mineralizable N. The amount of N taken up by the crop at the point of fertilization was also comparatively high in wheat following grass in 1995 and 1996 which indicates 62 Table 2-2: Assessment of optimum N rates for each rotation using the N balance model Rotation Mineralizable Soil N 11 Nt Plant N uptake ¶ Recommended N rate kg ha-1 On-farm Trials 1994 Clover-wheat Corn-wheat Grass-wheat 94.9 22.9 106.5 84.4 18.4 19.0 18.0 38.8 20.0 163 157 157 36.1 25.8 24.9 25.6 31.1 38.6 166 190 145 60.4 74.4 116.0 26.6 26.1 Oat-wheat Clover-wheat 1995 Clover-wheat Corn-wheat Grass-wheat 72.2 53.2 91.6 1996 Clover-wheat Corn-wheat Grass-wheat 33.3 15.1 26.7 42.8 187 177 114 55.1 33.7 17.6 193 59.7 43.1 14.3 179 Oat-wheat 63.2 13.1 6.5 217 Clover-wheat 85.3 14.4 31.7 168 Small Plot Trials 1996 1997 1. Soil mineralizable N kg ha' at Feekes 5 before fertilizer application 11 Residual N (NH4 -N + NO3-N) kg ha' at Feekes 5 before fertilizer application 11 Whole plant N uptake kg ha' at Feekes 5 before fertilizer application ImiRecommended N rate obtained by using model: N = 300 (N, + Nur, + N p) [1] 63 high N availability in grass rotation (Table 2-2). In small plots in 1996 and 1997, the soil inorganic N in the top 10 cm of soil at Feekes 5 was similar in oat and clover rotations (Table 2-2). In 1996, the soil mineralizable N in the top 10 cm of soil was also same for two rotations with average of 57 kg N ha-1. In 1997, however, wheat following clover had a higher soil mineralizable N compared to wheat following oat. A similar pattern was found for plant N uptake at Feekes 5 where in 1996 the N uptake was not significantly different between rotations but in 1997, clover-wheat had a significantly higher N uptake 32 kg N ha-' compare to 6 kg N ha1 for wheat following oat (Table 2-2). Based on the proposed model recommended N rates ranged from 114 to 217 kg N Grain Yield Response The grain yields for each site-year are summarized in Table 2-3. There was a significant yield response to fertilizer N in every trial. In all rotations, a significant yield increase occurred with the first increment of N fertilizer. The effects of additional N varied among rotations and years. This variation in yield response to added fertilizer N among rotations and years may have been due to the factors such as soil types and texture, water availability, soil nutrient levels and weather. Grain yield responded curvilinearly to applied N fertilizer as illustrated in Figs 2-1. Similar observations have been reported by Entz and Flower (1989) and others. The regression equations that best described grain yield response to N fertilizer at each site are given in Table 2-4. In the 1994, yields were high across all rotations (Table 2-2). The average yield across all rotations with no fertilizer was 6824 kg ha-I- a yield above the long term yield Table 2-3: Mean grain yield of on-farm trials for 1994-96 growing seasons. Previous crop Total NI applied Clover 1994 1995 Corn 1996 1994 kg ha"' Grass 1995 1996 1994 1995 1996 kg ha-1 0 5842a 4950a 4697a 7880a 5207a 4559a 6752a 3191a 4520a 56 7082b 5731b 6518b 9147b 6295b 6178b 9165b 5499b 5704b 112 7020bc 6138bc 7987c 9742c 7549c 7897c 9180bc 5853bc 6331bc 168 8076d 6258cd 8794d 10315d 7957cd 8111cd 10272d 6288cd 5915bcd 224 9082de 8251cde 5551bcde PLSD 949 517 513 493 975 690 935 925 720 CV % 6 4 3 2 7 6 5 9 5 P-value 0.008 0.004 0.000 0.008 0.009 0.003 0.001 0.000 0.00 I In 1994 and 1995 wheat following grass fertilizer N rates were 0, 67, 134, 201 kg N 65 11000 10000 9000 et 8000 7000 :T. a) 6000 5000 4000 3000 A 1994 0 1995 -X- 1996 2000 50 100 150 200 250 N rate (kg/ha) 11000 10000 9000 b 1 8000 7000 6000 I 5000 4000 3000 A-- 1994 0 1995 -- 1996 2000 0 50 100 150 200 250 N rate (kg/ha) Fig. 2-1 Yield response of (a) Clover-wheat (b) Corn-wheat (c) Grass-wheat rorations to fertilizer N rates of on -farm trials for the 1994-95 growing season 66 11000 10000 C 9000 t 8000 7000 6000 ;--: 5000 4000 3000 A 1994 0 1995 X-- 1996 2000 0 50 100 150 200 250 N rate (kg/ha) Fig. 2-1 (cont.) Yield response of (a) Clover-wheat (b) Corn-wheat (c) Grass-wheat rotations to fertilizer N rates of on -farm trials for the 1994-96 growing season 67 Table 2-4: Regression equations for nitrogen response curves of on-farm trials for the 1994-1996 growing season Rotation Regression equation R2 On-farm Trials 1994 Clover-wheat G = 5963 + 14.34 * N - 0.11 * N2 .66 Corn-wheat G = 7913 + 23.40 * N - 0.05 * N2 .78 Grass-wheat G = 6925 + 30.50 * N - 0.73 * N2 .98 Clover-wheat G = 4955 + 16.59 * N - 0.53 * N2 .77 Corn-wheat G = 5156 + 26.07 * N - 0.05 * N2 .84 Grass-wheat G = 3324 + 35.61 * N - 0.10 * N2 .92 Clover-wheat G = 4674 + 38.75 * N - 0.85 * N2 .82 Corn-wheat G= 4499 + 39.40 *N - 0.10 *N2 .77 Grass-wheat G = 4359 + 24.08 * N - 0.09 * N2 .67 Oat-wheat G = 2193 + 40.77 * N - 0.06 * N2 .97 Clover-wheat G = 4027 +36.80 * N - 0.07 * N2 .89 Oat-wheat G = 1171 + 43.38 * N 0.06 * N2 .97 Clover-wheat G= 3566 + 51.61 *N - 0.14 * N2 .94 1995 1996 Small Plot Trials 1996 1997 68 average for the region. The USDA Agriculture Statistics Service reported yields 15% above the long term average in western Oregon during 1994 (Sebastian, 1995). In the wheat following corn rotation, each increment of N fertilizer significantly increased yields (p < 0.05). The high response to N fertilizer for wheat following corn could be because corn provided a large quantity of a residue with a high C:N ratio. Echeverria et al. (1992) also found that wheat after corn responded most to N fertilizer as compared to wheat following soybean or sunflower. Wheat following clover in 1994 had lowest overall mean yields ranging from 5842 kg ha with no fertilizer to 8076 kg ha"1 with 168 N kg ha'. No significant difference was observed between the 56 and 112 kg N ha-1- treatments (Table 2-3). Current western Oregon fertilizer recommendations for wheat following clover are less than for wheat following row crops, but varies depending on how vigorous the clover crop was. A less than average stand of clover would result in less available N for the subsequent wheat crop. The check yield for wheat following grass in 1994 was 6752 kg ha' (Table 2-3). As was observed for the wheat following clover rotation, there was not a significant difference between treatments of 56 and 112 N kg ha."1 (Table 2-2). In 1995, response to N fertilizer for wheat following corn was similar to 1994 observations. Yield increased significantly with each increment of N fertilizer except at the highest N rate. Mean grain yields were, in general, greater in wheat following corn compared to wheat following clover or grass. The wheat following grass rotation had the lowest check yield (3191 kg ha-1) and the largest response to initial N increment. Adding 68 N kg ha-1 increased the yield by over 2300 kg ha-1. At the highest N fertilizer rate, the mean yield of wheat following clover and grass were the same, 6258 kg ha-1 and 6288 kg 69 ha-i, respectively. In addition, the yield increase from 56 to 112 N kg ha-1 was not significant for clover-wheat and grass-wheat rotations. As 1994-95 data suggested there many be a response to a higher N rate, a 224 N kg ha rate was included in 1995-96 experiments. No additional yield response was obtained for this higher rate in any rotation. Interestingly, the check yield for all three rotations was similar with an average value of 4592 kg ha-1. At higher N rates, mean yields of wheat following clover and corn were about the same, while yields of wheat following grass were less. In fact, the highest yield of wheat following grass was achieved with 112 N kg ha"'. Yield dropped when the fertilizer N was applied at the rates of 168 and 224 N kg ha-1 (Fig. 2-1 c). This reduction in yield at high N rates may be due to increase disease incidence, water stress, or to physiological reactions by the plant itself (Blade and Baker, 1991). No lodging occurred in any rotation. It was observed throughout the study that wheat following grass had relatively higher soil N availability (Table 2-2) and higher N uptake (data not shown) as compared to wheat following clover and wheat following corn. Determination of Nitrogen Rates for Optimum Grain Yield Figures 2-1 show yield as a function of applied fertilizer N for wheat following clover, corn and grasses. The yield data fit a quadratic model using applied N as an independent variable. Regression equations for yield response curves and their corresponding correlation coefficient (R2) are given in Table 2-4. 70 The amount of N fertilizer required at Feekes 5 to produce maximum yield (NMAX) in each rotation and years was determined using the N balance model. The NMAX rates predicted by this model were compared with actual N rates required to obtain maximum economic yields (NMEY). The amount of fertilizer N required for maximum economic yield were calculated from each response curve at each rotation site. The NMEY for any given response curve is the fertilizer rate where the first derivative of the response function equals the price ratio of N fertilizer to wheat [3]. Two N fertilizer/wheat price ratios were used in this study to calculate NMEY (Table 2-5). The difference between the two ratios was the price of wheat 0.14 $ per kg Ni and 0.16 $ kg for N2. The price of N was held constant at $ 0.60 per kg. The difference between the NMEY values (N1 and N2) was not significant (p > 0.8), consequently N1 is used in further comparisons. In 1994, the NMEY rates for wheat following clover and wheat following corn exceeded the largest N fertilizer rate used in study, therefore, the highest N rate of 168 N kg ha-1 was considered as NMEY rate. The comparison of NMAX and NMEY values are given in Table 2-5. At on-farm sites in 1994, the model underestimated NMEY by 5 N kg ha-1, 11 N kg ha-1 and 18 N kg ha-1 for wheat following clover, corn and grass, respectively. In 1995, the model overestimated N need in the clover rotation by 54 N kg ha-1. This was the largest difference observed in this study. In 1995, corn and grass rotation NMAX and NMEY rates were very close. In 1996, good agreement was found between NMAX and NMEY values for all three rotations with the largest difference only 7 N kg ha-1. 71 Table 2-5.Comparison of recommenced and actual N rates for maximum economic yield Rotation Yield at N t rate Actual N /trate for recommended recommended Max. Econ. Yield N rate by model NI N2 kg ha"' On-farm Trials 1994 Clover-wheat 7994 163 168 168 Corn-wheat 10216 157 168 168 Grass-wheat 9908 157 175 182 Clover-wheat 6258 166 112 121 Corn-wheat 8007 190 196 200 Grass-wheat 6287 145 147 152 Clover-wheat 8951 187 190 196 Corn-wheat 8290 177 170 175 Grass-wheat 5956 114 107 112 Oat-wheat 7953 193 200 200 Clover-wheat 9154 189 179 200 Oat-wheat 7915 217 200 200 Clover-wheat 8145 168 162 165 1995 1996 Small Plot Trials 1996 1997 t Recommended N rate calculated by using equation [1]. II N rate to obtain maximum economic yield. Ni and N2 are the rates where the price of wheat was set equal to $0.14 and $0.16 per kg, respectively, and price of N fertilizer was $0.60 per kg. 72 Average NMAX and NMEY rates, 174 and 178 N kg ha-1, respectively, were highest for wheat following corn. Due to high soil N, low average NMAX and NMEY rates (141 and 143 N kg ha-1) were observed for wheat following grasses. A good correlation between NMAX and NMEY ( r = 0.77) was observed across rotations and years. In small plot trials the N fertilizer rates predicted by the model were also fairly closed to the N rates required to obtain maximum economic yields. In 1996 and 1997, due to high response of grain yield to N fertilizer (Fig. 2-2), the NMEY rates for wheat following oat exceeded the largest N fertilizer rate, therefore, high rate of 200 was considered as NMEY rates. In 1997, NMAX and NMEY rates were very similar for both rotations. Wheat following oat had higher NMAX and NMEY rates- 35 kg N ha.-1 more. Model Validation In 1996-97, N rate trials were conducted to further check the validity of the proposed model. Recommended N rates and subsequent yield and grain protein concentration are given in Table 2-6. The recommended fertilizer N rates were 112 N kg ha-1 and 168 kg N haTI for wheat following grass and corn, respectively. Cooperating growers assisted in selecting other N treatments in these trials. Bracketing treatments were chosen at the grass site, while lower rates were considered at the corn site. In both rotations, the mean yield for the recommended rate was higher than the other two treatments used in the experiment (Table 2-4). In the grass rotation, mean yield dropped at the higher fertilizer N rate and there was no significant difference in yield between the high and low fertilizer N rate treatments. Soil analyses also revealed high 73 10000 9000 8000 7000 6000 5000 4000 3000 2000 1000 50 0 100 150 200 150 200 N rate (kg/ha) 10000 9000 (B) 1997 8000 t 7000 -c: 5000 -7)1 ---Nct 6000 4000 3000 2000 1000 -r 0 50 100 N rate (kg/ha) mr Oat-wheat --c Clover-wheat Fig. 2-2 Yield response of clover-wheat and oat-wheat rotations to fertilizer N rates (A) 1996 and (B) 1997 of small research plots trials 74 Table 2-6: Main yield and protein content for model validation trials conducted during 1996-97 winter wheat growing season N rate Yield (Kg ha -1) (Kg ha-1) Protein content Grass-wheat rotation 84 4651a 9.8a 112 t 5168b 9.7ab 140 4875ab 10.5c 0.15 CV (%) 381 2 P-value .004 .000 84 6476a 8.0a 132 7237b 8.7b 168 t 7946c 9.5c 577 0.56 PLSD 1 Corn-wheat rotation PLSD CV (%) P-value 3 .005 3 0.01 1. Recommended N rate by using model [1] residual N (NI-14-N and NO3-N) at the end of the season (data not shown). The plant N uptake at the end of the season was also higher for wheat following grass compared to wheat following corn (data not shown). Similar results were observed for wheat following grass on-farm trials in 1996 where mean yield dropped at higher fertilizer N rates, while total biomass and grain protein content increased significantly as higher fertilizer N was added (Fig. 2-3). Costa and Kronstad (1994) reported that the redistribution of N from 75 vegetative plant growth accounted for at least 50 % of grain protein N. Higher levels of biomass observed in the study due to higher levels of N, could potentially increase the amount of N to be redistributed which contributes to a higher grain protein N concentration. In the wheat following corn rotation each increment of N fertilizer significantly increased grain yield. These results are similar to observations for wheat following corn in 1994-96 in on-farm trials (Table 2-2). Conclusions Optimum N rates predicted by our proposed model were closely related to the N rates required to obtain maximum economic yield. In 1996-97, the model validation experiment also gave promising results. This study overall shows that the model appears to accurately assess field-specific optimum fertilizer N status and that it can be used to make fertilizer N recommendations for the region. The response of winter wheat to fertilizer N varied greatly among rotations and years in this study. Wheat following corn rotation was highly responsive to added fertilizer N while wheat following grass was less responsive. Wheat following grasses had high soil supplied N which depressed the yield even at moderately high fertilizer N rates. This finding contradicts conventional wisdom that suggests higher N rates are needed following grasses. This study documents a difference between perennial grasses and cereals grain crops in rotation. The average yield with no fertilizer for wheat following corn and wheat following legumes was about the same. 76 25000 12.5 -12 20000 11.5 11 to 15000 -10.5 10000 10 9.5 5000 -9 1 0 50 100 I 150 8.5 I 200 250 N rate (kg/ha) Biomass 0-- Grain 0--- Protein Fig 2-3: Total biomass, grain yield and protein content as influenced by fertilizer N for wheat following grass in 1996 growing season. 77 Further Recommendations Research efforts should be directed to further evaluate the validity of the model over a range of soil and crops. Further research on N mineralization in soils following grasses is also needed. 78 References Azam, F., F. W. Simmons, and R. L. Mulvaney. 1993. Mineralization of plant residues and its interaction with native soil N. Soil Biol. Biochem. 25:1787-1792. Azam, F. 1990. Comparative effect of organic and inorganic nitrogen sources applied to a flooded soil on rice yield and N availability. Plant Soil. 125:255-262. Azam, F., K. A. Malik, and F. Hussain. 1986. Microbial biomass and mineralization, immobilization of nitrogen in some agricultural soils. Bio. Fertil. Soils. 2:157-163 Baethgen, W. E., and M. M. Alley. 1989. Optimum soil and fertilizer nitrogen use by intensively managed winter wheat. II . Critical levels and optimum rates of nitrogen fertilizer. Agron. J. 81: 120-125. Batey, T. 1977. Prediction by leaf analysis of nitrogen fertilizer required for winter wheat. J. Sci. Food Agric. 28: 275-278. Becker, F. A., and W. Aufhammer. 1982. Nitrogen fertilization and methods of predicting the N requirements of winter wheat in Federal Republic of Germany. Proc. Fert. Soc. 211:33-66. Beuerlein, J. E., E. S. Oplinger, and D. Reicosky. 1992. Mineral content of soft winter wheat as influenced by nitrogen fertilization and management. Commun. Soil Sci. Plant Anal. 23:455-467. Blade, S. F., and R. J. Baker. 1991. Kernel weight response to source-sink changes in spring wheat. Crop Sci. 31:1117-1120. Boquest, D. J., and C. C. Johnson. 1987. Fertilizer effect on yield, grain composition, and foliar disease of double crop soft winter wheat. Agron. J. 79:135-141. Breland, T. A. 1994. Enhanced mineralization and denitrification as a result of heterogeneous distribution of clover residue. Plant Soil. 166:1-12. Bundy, L. G., M. A. Schmitt, and G. W. Randall. 1992. Predicting N fertilizer need for corn in humid regions: Advances in the upper Midwest. p. 73-89. In B.R. Bock and K. R. Kelly (ed.) Predicting N fertilizer need for corn in humid regions. Bull. Y-226 Natl. Fertilizer and Environ. Res. Muscle Shoals, AL. Burnett, C. A. 1975. The agriculture use of organic material in Brazil. FAO Soil Bulletin. 27:305-311. 79 Cochran, V. L., R. L. Warner, and R. I. Papendick. 1978. Effect of N Depth and application rate on yield, protein content, and quality of winter wheat. Agron. J. 70:964-968. Costa, J. M., and W. E. Kronstad. 1994. Association of grain protein concentration and selected traits in hard red winter wheat population in the Pacific Northwest. Crop Sci. 34:1234-1239. Collins, H. P., P. E. Rasumussen, and C. L. Douglas, Jr. 1992. Crop rotation and residue management effects on soil carbon and microbial dynamics. Soil Sci. Soc. Am.J.56:783-788. Cook, R. J. 1988. Biological control and holistic plant-health care in agriculture. Am. J. Alt. Agri. 3:51-62. Copeland, P. J., and R. K. Crookston. 1992. Crop sequence affects nutrient composition of corn and soybean growth under high fertility. Agron. J. 84:503-509. Cox, F. 1985. Survey of state soil testing laboratories in the United States. Soil Sci. Soc. Am. J. Madison, W I. Donohue, S. J. and D. E. Brann. 1984. Optimum N concentration in wheat grown in the coastal plan region of Virginia. Commun. Soil Sci. Plant Anal. 15: 651-611. Echeverria, H. E., C. A. Navarro, and F. H. Andrade. 1992. Nitrogen nutrition of wheat following different crops. J. Agric. Sci. 118:157-163. Engel, R. E., and J. C. Zubriski. 1982. Nitrogen Concentration in spring wheat at several growth stages. Commun. Soil Sci. Plant Anal. 13:531-534. Foth, D. H., and B. G. Ellis. 1988. The soil nitrogen cycle. In Soil Fertility. John Wiley & Sons. Ins. P. 69-73. Fox, R. H., and W. P. Piekielek. 1978. Field testing of several nitrogen availability indices. Soil Sci. Soc. Am. J. 42:747-750. Fox, R. H., and W. P. Piekielek. 1984. Relationship between anaerobically mineralized nitrogen, chemical indexes, and nitrogen availability to corn. Soil Sci. Soc. Soc. Am. J. 48:1087-1090. Franzluebbers, A. J., F. M. Hons, and D. A. Zuberer. 1994. Long-term changes in soil carbon and nitrogen pools in wheat management systems. Soil Sci. Soc. Am. J. 58:1639-1645 80 Fyson, A., and Oaks, A. 1990. Growth propotion of maize by legume soils. Plant and Soil. 122:259-266. Gelderman, R. H., W. C. Dahnke, and L. Swenson. 1988. Correlation of several N indices for wheat. Commun. Soil Sci. Plant Anal. 19(6):755-772 Goos, R. J., and L. Prunty. 1990. Yield variability and the yield goal decision. In J. L. Havlin (ed.) Proc. Of Great Plain Soil Fertility Conf., Denver, CO. 6-7 Kansas State Univ. Manhattan. Harper, J. E. 1984. Uptake of Organic Nitrogen Forms by Roots and Leaves. IN R. D. Hauck(ed.) Nitrogen In Crop Production. Am. Soc. Agron. Madison, WI. Keeney, D. R. 1982. Nitrogen availability indices. p 711-733. In A.L. Page et al. (Ed.) Methods of Soil Analysis. Part 2. Agron. Monrog. ASA and SSSA, Madson, WI. Large, E. C. 1954. Growth stages in cereals. Illustration of the Feekes scale. Plant Pathol. 3:128-129. Maples. R., J. G. Keogh, and W.E. Sabbe. 1977. Nitrate monitoring in cotton production in Loring- Calloway silt loam. Arkansas Agric. Exp. Stn. Bull. No. 825. Marumoto, T., J. P. Anderson, and K.H. Domsch.1982. Mineralization of nutrients from soil microbial biomass. Soil Biol. Biochem. 14: 469-475. Mary, B., and S. Recous. 1994. Measurement of nitrogen mineralization and immobilization fluxes in soil as a mean of predicting net mineralization. Eur. J. Agron. 3:291-300. McKenney, D. J., S. W. Wang., C. F. Drury, and W. I. Findlay. 1995. Denitrification, immobilization, and mineralization in nitrate limited and non limited residueamended soil. Soil Sci. Soc. Am. J. 59:118-124. Meisinger, J. J., R. F. Mulford, and J. S. Stangarone. 1987. Effect of residual N on winter wheat in Maryland. p. 209. In: Agronomy Abstracts. ASA, Madison, Wl. Memon, G. H., and G. H. Jamro. 1988. Influence of nitrogen fertilization on grain protein content and NPK content of straw in late sown wheat. Pak. J. Sci. Ind. Res 31:649-650. Neve, S.D., J. Pannier, and G. Hofman. 1994. Fraction of vegetable crop residues in relation to in situ N mineralization. Eur. J. Agron. 3:267-272. 81 Nielsen, D. C., and A. D. Halvorson. 1991. N fertility influence on water stress and yield of winter wheat. Agron. J. 83:1066-1070. Palm, C. A., and P. A. Sanchez. 1991. Nitrogen release from some tropical legumes as affected by lignin contents. Soil Biol. Biochem. 23:83-88. Peter C. S., M. M Alley, and Y.Z. Lei. 1993. Spring nitrogen on winter wheat: I. Farmerfield validation of tissue test-based rate recommendations. Agron. J. 85:1181­ 1186. Power, J. F., and J. Alessi. 1978. Tiller development and yield of standard and semidwarf spring wheat varieties as affected by nitrogen fertilizer. J. Agric. Sci. 90:97-108 Power, J. F., and J. W. Doran. 1984. Nitrogen Use in Organic Farming. In R. D. Hauck(ed.) Nitrogen in Crop Production. ASA. Madison, WI. Rauschkolb, R. S., T. L. Jackson, and A. I. Dow. 1984. Management of nitrogen in the pacific States. p. 765-777. In R.D. Hauck (ed.) Nitrogen in Crop Production. ASA. Madison, WI. Recous, S., J. M. Machet, and B. Mary. 1988. The fate of 15 N urea and ammonium nitrate applied to a winter wheat crop. Plant Science. 122: 215-224. Rice, C. W., and J. H. Havlin. 1994. Integrating mineralizable nitrogen indices into fertilizer recommendations. p. 1-14. In Soil testing. Prospects for improving nutrient recommendations. Soil Sci. Soci. Am. Specia. Pub. No. 40, Madison, WI. Robinson, F. E., D. W. Cudney, and W. L. Lehman. 1979. Nitrogen fertilizer timing, irrigation, protein, and yellow berry in durum whaet. Agron. J. 71:304-307. Roth, G. W., R. H. Fox, and H .G. Marshall. 1989. Plant tissue test for predicting nitrogen requirements of winter wheat. Agron. J. 81:502-507. Scharf, P. 1993. Development of field-specific spring N recommendations for winter wheat. Ph. D. Thesis. Virginia polytechnic Institute abs State Univ. Blacksburg, Virginia. Schepers, J. S., K. D. Frank, and C. Bourg. 1986. Effect of yield goal and residual soil nitrogen concentration on N fertilizer recommendations for irrigated maize in Nebraska. J. Fert. Issues. 3:133-139. 82 Schmitt, M. A., G. W. Randall, G. W. Rehm, and G. L. Malzer. 1991. Calibration and correlation of soil N test for corn's N recommendations. p. 212-221. In A report on field research in soils. Minn. Agric. Exp. Stn. Misc. Publ. 71. Sebastain, K. D. 1995. Nitrogen fertilization of winter wheat grown in four rotations in western region. M.S. Thesis. 101. pp. Oregon State University, Corvallis, OR. Smith, J. L. 1994. Cycling if nitrogen through microbial activity. In Advances in Soil Science. Lewis publishers. pp 91-119. Stanford, G. 1982. Assessment of soil N availability. p. 651-688. in F. J. Stevenson (ed). Nitrogen in Agriculture Soils. Agron. monogr. 22. ASA. CSSA, and SSSA, Madison, WI. Stanford, G., and J.O. Legg. 1984. Nitrogen and yield potential. In R. D. Hauck (ed.) Nitrogen in crop production. ASA, Madison, WI. p 263-293. Thicke, F. E., M. P. Russell., 0. B. Hesterman, and C. C. Sheaffer, 1993. Soil nitrogen mineralization indexes and corn response in crop rotation. Soil Science. 156:322­ 334. Vanotti, M. B., and L. G. Bundy. 1994. Frequency of nitrogen fertilizer carryover in the humid midwest. Agron. J. 86:881-886. Vaughan, B., K. A. Barbarick., D.G. Westfall, and P.L. Chapman. 1990. Tissue nitrogen levels for dryland hard red winter wheat. Agron. J. 82:561-565. Wani, S. P., W. B. McGill, and L. A. Robertson. 1991. Soil N dynamics and N yield of barley grown on Breton loam using biological fixation or fertilizer. Bio. Ferti. Soils. 12:1018. Wehrmann, J., and H. C. Scharf. 1979. De mineralstickstoffgehalt des bodens als mabstab fur den stickstoffdungerbedarf . Plant Soil. 52:109 Westfall, D. G. 1984. Management of nitrogen in the Mountain states. p. 751-763. In R. D. Hauck (ed.) Nitrogen in crop production. ASA. Madison, WI. Whitmore, A. P., and J. R. Groot. 1994. The mineralization of N from finely or coarsely chopped crop residues: measurement and modeling. Eur. J. Agron. 3:367­ 373. 83 CHAPTER 4 EVALUATION OF N UPTAKE PATTERNS IN SPRING AND WINTER WHEAT IN WESTERN OREGON Abstract An understanding of the above ground nitrogen uptake pattern for wheat (Triticum aestivum L.) is needed to facilitate nitrogen management. Evaluation of crop N uptake pattern is critical for high N fertilizer use efficiency. Maximum efficiency should be expected when plant N availability is synchronized with the period of rapid N uptake by the crop. The purpose of this study was to determine the N uptake pattern of spring and winter wheat grown in western Oregon. Data used in this study were obtained from three different trials: spring wheat rotation trials, small plot rotation trials, and unfertilized plots. In spring wheat rotation trials five spring wheat cultivars; Alpowa (soft white; SW), Pennawawa (SW), Treasure (SW), WB936R (hard red; BR), and Whitebird (SW) were used. Fertilizer N (16-16-16-4) at the rate of 140 kg ha-1 was shanked into the soil at the time of planting (April 4, 1997). An additional 194 kg ha' of fertilizer (46-0-0-0) was broadcast on Aril 27, 1997. Small plot rotation trials were established during the 1993-94 growing season at the Hyslop Experimental Farm of the Department of Crop and Soil Science, Oregon State University, Corvallis, Oregon. Five fertilizer treatments - 0, 50, 100,150 and 200 kg N ha' were used. Rotations include winter wheat following clover 84 and winter wheat following oat. In 1996, N uptake and dry matter yield of winter wheat were determined from unfertilized plots of Stephens wheat in a trial at Hyslop Experimental Station. Three 30 cm rows of above-ground plant tissue were randomly cut at approximately 15 days interval starting a Feekes 5 and continuing until maturity. In examining the N uptake pattern of spring and winter wheat it was observed that maximum N uptake for spring wheat was at approximately 1100 accumulated growing GDD, before Feekes 10. The maximum N uptake rate, 0.038 kg N GDD-1, occurred at 750 GDD. The peak N uptake was observed at May 30, approximately 35 days after Feekes 2. Nitrogen uptake in winter wheat was significantly affected by rotations. Significant differences for rate and time of maximum uptake between rotations were observed. The maximum rate of N uptake (0.4-0.5 kg N GGD-1) for wheat following clover was observed at approximately 1100 accumulated GDD, while the maximum rate of N uptake (0.1-0.2 kg N GGD-1) for wheat following oats was at 1300 GDD. 85 Introduction Evaluation of crop N uptake pattern is critical for high N fertilizer use efficiency. Timing of fertilizer N application to coincide with specific growth stages can maximize efficiency. High fertilizer N use efficiency is a key factor in achieving optimum cereal grain yield and reducing the opportunities for N losses by denitrification, leaching and volatilization. Maximum efficiency should be expected when plant N availability synchronizes with the stage of crop development that permits rapid N uptake (Olson and Kurtz, 1982). Better understanding the crop N uptake pattern for wheat in a particular environment is critical for improving N fertilizer management and adjusting timing of fertilizer application (Harper et al., 1987; Papastylianou et al., 1984). In the humid midAtlantic region of the US, Baethgen and Ally (1989) conducted a study to determine the pattern of winter wheat N uptake through the spring growing season and found that maximum daily N uptake rates were obtained in the period immediately after Zadoks GS 30 (Feekes 5), suggesting that this is when the highest efficiency of N fertilizer use could be expected. Nitrogen uptake by plants depends on several factors including nitrogen use efficiency of the plant (yield per unit of N supply), N application rate and timing, availability of N in the soil solution at the root surface, the state and morphology of roots, temperature and other climate-related factors (Karrou and Maranville, 1994; Bock, 1984). Total nitrogen content (the amount of nitrogen present in the aboveground portion of a plant) shows the plant's capability to accumulate nitrogen. Fiez et al. (1995) argued 86 that N use efficiency can be divided into two multiplicative components - N uptake efficiency and N utilization efficiency. Nitrogen efficiency illustrates the ability of a plant to take up supplied N while utilization efficiency shows the capability of plant to utilize N by increasing yield. Application of N fertilizer stimulates early vegetative growth. The addition of fertilizer N can increase vegetative growth and the amount of dry matter and N in crop at anthesis (McDonald, 1992). In wheat, nitrogen content per plant is positively correlated with total dry matter (Desai and Bhatia, 1978). Nitrogen uptake per unit N applied is greatest where yield response to N is high (Gauer, et al. 1992). Increase in N uptake due to fertilizer mainly depends on the N supplying capacity of the soil and the plants' ability to use available N. Time of fertilizer N application greatly affects the N uptake efficiency in wheat production systems. In high precipitation regions, spring applied N is superior to fallapplied N (Doll, 1962). Spring-applied N was more available to winter wheat and more profitable than fall or split-applied N in a semi-arid climate (Vaughan et al., 1990). In Kansas, Kelly (1995) found no significant yield increase from split N (fall + late winter) and split-spring N (fall + late winter + early spring) applications compared with single preplant or late winter topdress applications, regardless of previous crop. Johnston and Fowler (1991) observed that early N uptake and early growth was prevented by delaying spring N fertilization of winter wheat under dryland conditions. Type of fertilizer N also influences the N uptake and total dry matter yield of a wheat crop. Xingting and Below (1992) argued that the growth and yield of wheat is enhanced when plants are provided a 87 mixture of NO3-N and NH4-N compared to either form alone. They suggest that the growth and yield increase due to mixed N was because the mixed N increased the root number and branches which enhanced N uptake. One of the primary factors affecting N uptake is root morphology (Xingting and Below,1992). Luxova (1988) argued that even though the entire root system is involved in uptake, the majority of uptake occurs in the apical zones or axes of branches in young roots. Moorby and Besford (1983) found that the growth of lateral roots increased the rate of N uptake. Similarly, addition of fertilizer N affects growth and morphological development of roots. Tennant (1976) found that root length increased when N was applied to either part or all of a cereal root system. Nitrogen uptake closely follows the growth pattern and at a certain developmental stage, N uptake ceases and remobilization of N within the plant starts. Bauer et al. (1987) measured N uptake in hard red wheat aerial parts from three-leaf to kernel-stage and found that peak N uptake in leaves occurred during flag-leaf-extension through the boot stage. They also reported that about 70 % of the 112 kg N ha' found in spikes was translocated from leaves and stems. Baethgen and Alley (1989) observed that the total amount of N found in leaves and stems decreased with time during the spring, while spike N increased from the earliest stages of development until harvest. McNeal et al. (1968) reported that under an irrigation system, 64 % of N translocated from leaves, stems and chaff to grain of seven heard winter wheat varieties. Waldren and Flowerday (1979) evaluated the concentration of N in leaves, stems, and spikes and found that translocation accounted for over 70 % of N in grain of hard red winter wheat. Lal et al. (1978) also 88 reported that 58 % of grain N translocation from other parts of wheat crop. Among environmental factors, temperature is the most important element that affects N uptake in plants by regulating growth. Many researchers have reported a close relationship between accumulated heat unit (growing degree days) and phenological development in cereals (Buxton and Marten; 1989; Bauer et al., 1984; Klepper et al., 1982). Because of its precision as in estimator of growth rate, growing degree days (GDD) has been incorporated into N fertilizer management practices and crop development models. Bauer et al. (1984) investigated the utility of GDD to estimate spring wheat growth rate and stages and reported that precision of estimating growth rate and stages by GDD was superior to calendar days. The objective of this study was to determine the N uptake pattern of spring and winter wheat grow in western Oregon. This information will be summarized in a bulletin format for use by growers and field representatives in making decisions about wheat fertilization. 89 Materials and Methods Data were obtained from three different trials. Information about sampling dates, Feekes growth stage, and accumulated growing degree days (GDD) for each trial are shown in Table 3-1. Spring Wheat Rotation Experiment Nitrogen uptake and dry matter production in spring wheat were measured in an on-going rotation study in 1997. The experiment was conducted at the USDA-ARS Sustainable Grass Seed Production Research Project Coon Farm site located in Linn county, Oregon. This site is an untiled, poorly drained, low pH Dayton soil that is intermittently flooded during much of winter and early spring. The purpose of the this on­ going experiment is to determine the effect of perennial ryegrass residue management on rotation crops. In 1997, spring grains were grown in rotation. The five spring wheat cultivars used in this study were: Alpowa (soft white; SW), Pennawawa (SW), Treasure (SW), WB936R (hard red; HR), and Whitebird (SW). The wheat cultivars were planted on a 17 cm row spacing on April 4, 1997 using a conventional John Deere double disk opener drill as a no till planter. A seeding rate of 40 seeds/30 cm of row was used. The experimental design was a randomized complete block with four replications. Two 10-row wide strips of each cultivar were planted in each of four replicated blocks. Fertilizer N (16-16-16-4) at the rate of 140 kg ha.-1 was shanked into the soil at the time of planting. An additional 194 kg ha-1 of fertilizer (46-0-0-0) was broadcast on Aril 27, 1997. Aboveground plant samples were taken from randomly selected 1 m sections of row 90 Table 3-1: Sampling dates and corresponding Feekes growth stages and accumulated growing degree days for spring and winter wheat experimental sites. Experiment Sampling Date Feekes Stage GDD April 25 F2 218 May 13 F3 466 May 31 F6 764 June 22 F 10 1126 July 11 F 10.5 1432 August 1 F 11 1784 March 4 F4 961 May 5 F8 1455 July 25 F 11 2763 February 22 F4 999 May 2 F9 1614 July 25 F 11 3020 Spring Wheat 1997 Winter Wheat 1996 1997 approximately every 250 growing degree days starting from Feekes GS 1 and continuing until maturity. One sample was taken from each of the treatment subplots in a block and combined. Plant samples were dried at 70 °C in a forced air oven. Samples were then weighed and ground in a Wiley mill to pass a 1 mm mesh screen. Total N content was determined by combustion analysis. Nitrogen uptake in kg N ha."1 was calculated as product of total N content (%) in plant tissue times the biomass in kg ha.-1 divided by 100. 91 Small Plot Research Station Trials Nitrogen uptake and biomass data for winter wheat were obtained from fertility trials established during the 1993-94 growing season at the Hyslop Experimental Farm of the Department of Crop and Soil Science, Oregon State University, Corvallis, Oregon. The experiment is arranged as a split plot design with four replications having rotation as main plot and fertilizer treatments as sub-plots. Main plots are 12m X 45m, while sub plot fertilizer treatment size is 12m X 9m. Five fertilizer treatments - 0, 50, 100,150 and 200 kg N ha-1 - are used. Rotations include winter wheat following clover and winter wheat following oat. Plant tissue samples were collected at three different growth stages in 1996 and 1997 and used for dry matter yield and nitrogen uptake determination. Data used in this study were obtained from two N rate treatments; 0 and 100 kg N ha."1. Plant samples were collected at Feekes 5 (spring fertilization), at Feekes 9 (flag leaf), and at Feekes 11 (maturity). At Feekes 5 and Feekes 9, plant samples were taken from one m of row. At maturity, four plant samples of 1.5 meter of row were cut from drill strips in each plot. Heads were cut, threshed and weighed. Grain and straw were analyzed separately for their nitrogen content by combustion analysis. Plant N uptake (kg N ha.-1) was calculated by multiplying the N concentration in the tissue by dry matter production (kg DM ha-1). N uptake from Unfertilized Plots In 1996, N uptake and dry matter yield of winter wheat were determined from unfertilized plots of Stephens wheat in a trial at Hyslop Experimental Farm of the 92 Department of Crop and Soil Science, Oregon State University, Corvallis, Oregon. Three 30 cm rows of above-ground plant tissue were randomly cut at approximately 15 days intervals starting a Feekes 5 and continuing until maturity. All three plant samples were mixed. All plant samples were dried at 70 °C in a forced air oven. Plant samples were than weighed and ground in a Wiley mill to pass a 1 mm mesh screen. Total N content was determined by combustion analysis. Nitrogen uptake (kg ha') was calculated as product of total N content (%) in plant tissue times the biomass kg ha-1 divided by 100. Statistical Analysis In the spring wheat trial, sampling date was treated as a as main plot and cultivars as subplots. In the Hyslop Farm rotation trial, treatment variables included rotation as main plot and fertilizer N treatments as subplot. No statistical analysis was used for the unfertilized wheat trial. A logistic model was used to describe biomass accumulation and N uptake as a function of time. The model used for fitting the plant growth and nitrogen uptake is given in equation [1]. The parameters of the model describing dry matter accumulation and accumulative N uptake were estimated by using Statgraphic Dos ver. 2.0. K 1+ [ (K No)] No [I] exp rt 93 Where: Nt = Biomass accumulation or N uptake at time t K = Maximum Biomass accumulation or N uptake No = Initial or minimum biomass accumulation or N uptake r = Rate of increase (slope factor) in biomass accumulation or N uptake t = Time (growing degree days) exp = Base of natural log, 2.71828 The first derivative of equation [1] was taken to determine a rate function dN/dt [2]. The time of maximum biomass accumulation and maximum N uptake were estimated by plotting dN/dt. dN dt - rN (1- N) K [2] Growing degree days were calculated from daily maximum and minimum temperatures by Eq. [3]: GDD = [(T mix + Tmto)/2] Where, T = Daily maximum temperature ( °C) Ttnti, = Daily maximum temperature ( °C) Tb = Based temperature with 0 °C Tb [3] 94 Results and Discussion Spring wheat Significant interaction between biomass accumulation and sampling date was found (p< 0.002) which indicates that the pattern of biomass accumulation of five cultivars varied with date of sampling. The mean values for biomass accumulation, Feekes growth stages and their corresponding accumulated GDD are shown in Table 3-2. Biomass accumulation followed a sigmoidal response (Fig. 3-1). Maximum biomass yield for all five cultivars was observed at approximately 1432 GDD or Feekes 10.5. Biomass accumulation leveled off beyond 1432 GDD. Each of five cultivars accumulated approximately the same amount of dry matter until 1125 GDD or Feekes 10. A difference in biomass yield among cultivars was observed at 1432 and 1784 GDD, Feekes 10.5 and Feekes 11, respectively (Fig. 3-1). At 1432 GDD, the difference for biomass yield between Pennawawa and Alpowa was 2595 kg ha' (Table 3-2). The interaction effects of cultivar and sampling date for N uptake were not significant (p> 0.2) indicating that the pattern of N uptake for all cultivars was the same and the magnitude of N uptake of all cultivars was independent of sampling date. Significant differences among cultivars were observed for N uptake (p < 0.001). The mean values of N uptake for each cultivar are given in Table 3-2. Sampling dates and their corresponding GDD are shown in Table 3-1. N uptake pattern also showed a sigmodal response (Fig. 3-2). The coefficient of determination (R2) for the model ranged from 0.93 to 0.96 indicating a highly significantly fit of the model. Table 3-2: Accumulative biomass yield and nitrogen uptake of five spring wheat cultivars for 1997 growing season. Sampling Time (Growing Degree Days) 218 467 763 1125 1433 1784 F2 F3 F6 F10 F 10.5 F 11 Variety Biomass N uptake Biomass N uptake Biomass N uptake Biomass N uptake Biomass N uptake Biomass N uptake kg ha' Alpowa 52 2.5 618 25 3130 76 9126 115 11379 113 11264 117 Pennawawa 66 3.2 668 30 3573 93 8588 152 13797 155 13859 155 Treasure 68 3.2 790 28 3297 79 7935 146 13560 151 14167 152 WB963R 80 3.9 909 35 4653 105 9729 134 12427 129 12433 134 White Bird 58 2.7 697 27 3229 73 8682 119 11939 129 12122 128 96 16000 14000 03 -a) 12000 ..­ i'd 10000 E .2 ..c. a) 8000 i 6000 tE 4000 U 2000 0 100 m Alpowa 400 700 1300 1000 Time (GDD) -IN- Pennawawa v Treasure 1600 -0- VVB963R 1900 v White Bird Fig 3-1: Comparison of five spring wheat cultivars for biomass accumulation in 1997 growing season 97 The N uptake of all cultivars was rapid at early growth stage of the crop until the crop accumulated 1000 GDD (Fig 3-2). After accumulation of 1000 GDD, N uptake become slow and virtually there was no N uptake from 1120 to 1784 accumulated GDD, Feekes 10 to Feekes 11, respectively, for all cultivars. Rapid N uptake at early growth stages was associated with increase in biomass since it is well known that in wheat N uptake and dry matter accumulation is positively correlated (Austin et al., 1977). Maximum N uptake for all cultivars except WB963R was observed at approximately 1100 GDD, before Feekes 10. For WB963R, maximum N uptake was obtained at approximately 900 GDD (Fig. 3-2). Similar results were reported by Bauer et al. (1987) were they found that for spring wheat peak N uptake occurred during flag-leaf extension (Feekes 10) to boot stage (Feekes 10.1). At Feekes 10, greatest N uptake was observed for Pennawawa and Treasure, 152 and 146 kg N ha', respectively. The lowest plant N uptake at Feekes 10 was observed for Aplowa and White Bird, 115 and 119 kg N ha-1 (Table 3-2). Over all, Pennawawa had the highest and Alpowa had the lowest N uptake throughout the growing season. Average N uptake rate (kg/ha/GDD) of all five cultivars is shown in Fig. 3-3. Plant N uptake was very high from 218 GDD (first sampling date) to 750 GDD. The maximum N uptake rate, 0.038 kg N GDD-1, occurred at 750 GDD (Fig. 3-3). After accumulation of 750 GDD, the rate of plant N uptake declined and the crop almost stopped taking N at 1100 GDD. The window of N uptake for spring wheat on a calendar day basis was about 50 days, starting April 25 and ending June 15. However, the peak N uptake was compressed 98 160 Tzi ---al" 140 x x0 120 sa,100 Z 80 0 > i 60 5 40 U 100 1= Alpowa 400 700 1000 1300 Time (GDD) 1600 --m Pennawawa ---nz-- Treasure 0 WB963R 1900 v White Bird Fig 3-2 Comparison of five spring wheat cultivars for nitrogen uptake in 1997 growing season. 99 0.25 0.04 -0.20 e0.03 g Pr -0.15 t 0.02 -0.10 = 0 01 c--1- -0.05 Z 0.00 0.00 100 10 500 900 1300 1700 Time (ccumulative GDD from planting) 40 65 90 110 Time (calender days from planting) Fig 3-3: Average nitrogen uptake and biomass accumulatoin rates of spring wheat in 1997 growing season rn 100 into a 30 days period. Winter Wheat A similar pattern for N uptake in winter wheat was observed for both 1996 and 1997 growing years. Nitrogen uptake was significantly affected by rotations. Wheat following clover had greater N uptake at all sampling dates for both levels of N over two years as compared to wheat following oat (Fig. 3-4). Nitrogen uptake of wheat following clover receiving 100 kg ha"' was very rapid at early growth stages compared to wheat following oat. In wheat following clover, maximum N uptake, 130 kg N ha.-1, was observed at approximately 1350 GDD, prior to Feekes 9 growth stage. There was no change in N uptake was observed after 1350 GDD. In wheat following oat, the crop continued N uptake after Feekes 9 growth stage in both years. In 1996, maximum N uptake, 105 kg N ha-1, was observed at approximately 1750 accumulated GDD, while in the 1997 growing season, the peak N uptake of 100 kg N ha' for wheat following oats was at 2180 GDD. The first order derivative sigmoidal curve describing N uptake rate clearly shows the significant differences for rate and time of maximum uptake between rotations where 100 kg ha-1 N fertilizer was applied (Fig. 3-5). The wheat following clover had significantly higher N uptake rates in both growing years compared to wheat following oat. The maximum N uptake rate of both rotations dropped in the 1997 growing year. In wheat following clover, maximum rates of N uptake of 0.5 and 0.4 kg N ODD' were observed in 1996 and 1997 growing seasons, respectively, at approximately 1100 GDD. 101 z (L) 80 60 40 (..) 20 0 900 (A) 1400 2900 2400 1900 Time (GDD) 160 100-N _1140 -61) a 120 100-N -g 100 z 80 60 0 -N 40 0 0-N e) 20 0 (B) 1 900 1400 1900 2400 Time (GDD) --cr Clover-wheat I 2900 3400 Oat-wheat Fig 3-4 Nitrogen uptake in winter wheat as influenced by different rotations and fertilizer N rates in (A) 1996 and (B) 1997 at Hyslop Farm. 102 0.5 1996 A 0.4 t 0.3 a) 0.2 F4 'Alt 0.1 Fll F8 0 800 1200 1600 2000 2400 2800 114 160 196 224 249 266 (A) 0.5 0.4 Clover-wheat t 0.3 a) Oat-wheat 0.2 u. z 0.1 0 800 (B) 107 1200 1600 2000 2400 2800 Time (ccumulative GDD from planting) 168 209 235 259 283 Time (calendar day from planting) Fig 3-5 Nitrogen uptake rate in winter wheat receiving 100 kg N ha -1 as influenced by different rotations in (A) 1996 and (B) 1997 at Hyslop Farm 103 After accumulation of 1100 GDD, the rate of plant N uptake declined sharply and crop almost stopped taking N at 1400 GDD. The maximum rate of N uptake of 0.2 and 0.1 kg N GDD-1 for wheat following oats was observed in 1996 and 1997 growing seasons, respectively, at approximately 1300 GDD. The rate of N uptake decreased after 1300 GDD and ceased at 2000 GDD. Though uptake rates varied by years, growing degree days to maximal uptake was similar for each rotation over years. The effect of added fertilizer N on N uptake and biomass yield of winter wheat was also examined in this study. The mean values of each variable are given in Table 3-3. N uptake by winter wheat was significantly influenced by rotations and added fertilizer (p < 0.01). In 1996, the interaction effects of rotation and N fertilizer treatments for N uptake were not significant (p> 0.2), but in 1997, significant interaction was observed between rotation and N fertilizer treatments. In 1997, the influence of previous crop on N uptake was greater relative to 1996 especially for wheat following clover where N uptake increased in 1997 compare to wheat following oats. This indicates that N was more readily available following clover over time. Nitrogen uptake was significantly higher following clover than following oats at each level of applied N fertilizer, Over all, in both rotations, N uptake increased almost linearly with added fertilizer N. No significant interaction between rotation and fertilizer N treatments was found for biomass yield. In 1996, the rotation effect was not significant, but in 1997 the difference between the two rotations for biomass yield was significant (p< 0.04) perhaps indicating that with time the difference between the two rotation is becoming more pronounced. A significant increase in biomass yield was observed at the first increment of Table 3-3 Biomass yield, nitrogen uptake, and carbon to nitrogen ration of winter wheat of small plot research experiment for 1996 and 1997 growing seasons. Previous crop Fertilizer N Oat-wheat Biomass kg ha-1 1996 N uptake 1997 1996 1997 Clover-wheat C:N ratio 1996 1997 Biomass 1996 kg ha' N uptake 1997 C:N ratio 1996 1997 1996 1997 kg ha-1 0 5660 2965 44 20 171 181 7140 9420 59 57 175 274 56 9292 10047 64 54 201 336 15951 16726 120 94 194 282 112 15836 17245 109 101 209 284 15979 20814 133 143 160 189 168 16734 18620 131 140 152 262 17603 21776 135 168 165 153 224 19741 19525 156 174 125 139 21976 21479 215 225 103 104 4506 3151 38.4 25.5 24.4 64.0 4506 3151 38.4 25.5 24.4 64.0 21 13 22 14 10 19 21 13 22 14 10 19 PLSD (0.05) CV (%) 105 N fertilizer for both rotations over years (Table 3-3). In 1996, increasing N fertilizer from 100 to 150 kg N ha-1 did not increased the biomass yield in either rotation. In 1997, biomass yield of wheat following clover was significantly higher than wheat following oats. In wheat following clover, increasing N fertilizer from 100 to 200 kg ha-1 did not significantly increased biomass yield (Table 3-3). The biomass 1997 yield of wheat following clover with no fertilizer N was 6500 kg ha1 higher than the biomass yield of wheat following oats. Nitrogen uptake and biomass yield in the 1996 unfertilized Stephens plots showed the same pattern as it was observed in the Hypslop farm rotation experiment (Fig. 3-6). At early growth stages, Feekes 3 to Feekes 5, N uptake was rapid. Maximum N uptake was achieved at Feekes 8 (May 8). There was litte change in total N uptake from Feekes 8 to maturity. Biomass yield increased linearly from Feekes 3 to Feekes 8. Highest increase in biomass yield was observed at Feekes 8 to Feekes 10.1. Maximum biomass yield was obtained at Feekes 10.1 and beyond that biomass accumulation leveled off. Conclusions The amount of N taken up by spring wheat was different among cultivars. The pattern of plant N uptake was same for all five spring cultivars. Maximum N uptake for all five cultivars was observed at approximable 1100 accumulated growing GDD, before Feekes 10. The maximum N uptake rate, 0.038 kg N GDD-1, occurred at 750 GDD. On calendar day bases, the range of N uptake was about 50 days, starting from April 25 and ending at June 15. The peak N uptake was observed at May 30, approximable 35 days 106 10000 80 70 8000 60 50 6000 'Ia.' 40 4000 too 30 ..4A1 20 2000 10 0 0 3-8 3-23 4-8 4-25 5-13 6-2 6-26 7-13 Sampling Date o Biomass N Uptake Fig. 3-6: Accumulative biomass yield and nitrogen uptake of winter wheat from unfertilized plot during 1996 growing season. 107 after Feekes 2. Nitrogen uptake in winter wheat was significantly affected by rotations. Wheat following clover had greater N uptake at all sampling dates for both levels of N over two years as compare to wheat following oat. Significant differences for rate and time of maximum uptake between rotations were also observed. The maximum rate of N uptake for wheat following clover was observed at approximable 1300 accumulated GDD, while the maximum rate of N uptake for wheat following oats was at 2000 GDD. 108 References Austin, R.B., M. A. Ford, J. A. Edrich, and R. D. Blackwell. 1977. The nitrogen economy of winter wheat. J. Agric. Sci. 88:159-167. Bauer, A., A. B. Frank, and A. L. Black. 1984. Estimation of spring wheat leaf growth rates and anthesis from air temperature. Agron. J. 76: 829-835. Bauer, A., A. B. Frank, and A. L. Black. 1987. Aerial parts of hard red spring wheat. II. Nitrogen and phosphorus concentration and content by plant development stages. Agron. J. 79:852-858. Baethgen, W. E., and M. M Alley. 1989. Optimizing soil and fertilizer nitrogen use by intensively managed winter wheat. 1. Crop nitrogen uptake. Agron. J. 81:116­ 120. Bock, B. R. 1984. Efficient use of nitrogen in cropping systems. p. 273-294. In R.D. Hauck et al. (ed.) Nitrogen in crop production. ASA, CSSA, and SSSA, Madison, WI. Buxton, D. R., and G. C. Marten. 1989. Forage quality of plant parts of perennial grasses and relationship to phenology. Crop Sci. 29:429-435. Desai, R.M., and C.R. Bathia. 1978. Nitrogen uptake and nitrogen harvest index in durum wheat. Euphytica. 27:561-566. Doll, E. C. 1962.Effect of fall-applied nitrogen fertilizer and winter rainfall on yield of wheat. Agron. J. 54:471-473. Fiez, T. E., W. L. Pan., and B. C. Miller. 1995. Nitrogen use efficiency of winter wheat among landscape positions. Soil Sci. Am. J. 59: 1666-1671. Gauer, L. E., C. A. Grant, D. T. Gehl, and L. D. Bailley. 1992. Effects of nitrogen fertilizer on grain protein content, nitrogen uptake, and nitrogen use efficiency of six spring wheat cultivars, in relation to estimated moisture supply. Can. J. Plant Sci. 72: 235-241. Harper, L. A., R. R. Sharp, G. W. Langdale, and J. E.Giddens. 1987. Nitrogen cycling in wheat crop: Soil, plant and aerial nitrogen transport. Agron. J. 79:965-973. Johnston, A. M., and D. B. Fowler. 1991. No-till winter production: response to spring applied N fertilizer form and placement. Agron. J. 83: 722-728. 109 Karrou, M., and J. W. Maranville. 1994. Response of wheat cultivars to different soil nitrogen and moisture regimes: II. Nitrogen uptake, partitioning and influx. J. Plant Nut. 17: 745-761. Kelly, K. W. 1995. Rate and timing of nitrogen application for wheat following different crops. J. Prod. Agric. 8:339-345. Klepper, B., B. R. Rickman, and C. M. Peterson. 1982. Quantitative characterization of vegetative development in small grain cereals. Agron. J. 74:789-792. Lal, P., G. G. Reddy, and M. S. Modi. 1978. Accumulation and redistribution pattern of dry matter and N in triticale and wheat varieties under water stress conditions. Agron. J. 70: 623-626. Luxova, M. 1988. The participation of the primary maize root in the assimiluation of NH4-N ions. Plant Soil. 111:187-189 McDonald, G. K. 1992. Effects of nitrogenous fertilizer on growth, grain yield and grain protein concentration of wheat. Aust. J. Agric. Res. 43: 949-967. McNeal, F. H., M. A. Berg, and C. A. Watson. 1968. Nitrogen and dry matter in five spring varieties at successive stages of development. Agron. J. 58:605-608. Moorby, J., and R. T. Besford. 1983. Mineral nutrition and growth. p. 481-527. In A. Lauchli and R.L. Bieleski (ed) Encyclopedia of plant plant physiology. Vol. 5B. Springer-Verlag. Berlin. Olson, R. A., and L. T. Kurtz. 1982. Crop N requirements, utilization and fertilization. p. 567-604. In F.J. Stevenson (ed.) Nitrogen in agricultural soils. Agron. Monogr. 22. ASA, CSSA, and SSSA, Madison, WI. Papastylianou, I., R. D. Graham, and D. W. Puckridge. 1984. Diagnosis of the nitrogen status of wheat at tillering and prognosis for maximum grain yield. Commun. Soil Agron. 22:651-688. Tennant, D. 1976. Root growth of wheat. I. Early patterns of multiplication and extension of wheat roots including effects of level of N, P, and K. Aust. J. Agric. Res. 27:183-96. Vaughan, B., D. G. Westfall, and K. A. Barbarick. 1990. Nitrogen rate and timing effects on winter wheat grain yield, grain protein, and economics. J. Prod. Agric. 3:324­ 328. 110 Waldren, R. P., and A. D. Flowerday. 1979. Growth stages and distribution of dry matter, N, P, and K in winter wheat. Agron . J. 71:391-397. West, C. P., D. W. Walker, R. K. Bacon, D. E. Longer, and K. E. Turner. 1991. Phonological analysis of forage yield and quality in winter wheat. Agron. J. 83:217-224. Xingting, W., and F. E. Below. 1992. Root growth, nitrogen uptake, and tillering of wheat induced by mixed-nitrogen source. Crop Sci. 32: 997-1002. 111 CONCLUSIONS Soil mineralizable nitrogen estimated by the 7-day anaerobic incubation method correlated well with field-measured N availability. A linear relationship was found between soil mineralizable N test results and soil supplied N. A more than four fold increase in soil supplied N values, 20 to 110 kg N 11', was observed when the soil mineralizable N test values increased from 14 to 29 mg N kg"'. No consistent rotation effect was observed for wheat following clover or corn while rotation effects were obvious for wheat following oat and wheat following grass. Wheat following oat had very low soil mineralizable N test values while wheat following grass had comparatively high soil mineralizable N test values. Our results indicate that soil mineralizable N test values estimated by the 7-day anaerobic incubation method satisfactorily predict approximate soil N availability in western Oregon soils and that results can be used to adjust fertilizer N requirements in western Oregon. Response of winter wheat to fertilizer N varied greatly among rotations and years in this study. Wheat following corn was highly responsive to added fertilizer N while wheat following grass seed was less responsive. Wheat following grass seed had high soil supplied N which depressed the yield even at moderate fertilizer N rates. This finding contradicts conventional wisdom suggesting that higher N rates are needed following grass. This study documents a difference between perennial grasses and cereal grain crops in rotation. 112 In examining the N uptake pattern of spring and winter wheat it was observed that maximum N uptake for spring wheat was at a approximately 1100 accumulated growing GDD, before Feekes 10. The maximum N uptake rate, 0.038 kg N GDD-1, occurred at 750 GDD. The peak N uptake was observed at May 30, approximately 35 days after Feekes 2. Nitrogen uptake in winter wheat was significantly affected by rotations. Significant differences for rate and time of maximum uptake between rotations were observed. The maximum rate of N uptake (0.4-0.5 kg N GGD-1) for wheat following clover was observed at approximately 1100 GDD, while the maximum rate of N uptake (0.1-0.2 kg N GGD-1) for wheat following oats was at 1300 GDD. 113 BIBLIOGRAPHY Aiman, N. 1992. Evaluation of the chemical methods for estimating potential mineralizable organic nitrogen in soils. M.S. Thesis. Kansas State Univ., Manhattan. Austin, R.B., M.A. Ford, J.A. Edrich, and R.D. Blackwell. 1977. The nitrogen economy of winter wheat. J. Agric. Sci. 88:159-167. Azam, F., K. A. Malik, and F. Hussain. 1986. Microbial biomass and mineralization, immobilization of nitrogen in some agricultural soils. Bio. Fertil. Soils. 2:157-163 Azam, F. 1990. Comparative effect of organic and inorganic nitrogen sources applied to a flooded soil on rice yield and N availability. Plant Soil. 125:255-262. Azam, F., F. W. Simmons, and R. L. Mulvaney. 1993. Mineralization of plant residues and its interaction with native soil N. Soil Biol. Biochem. 25:1787-1792. Baethgen, W. E., and M.M Alley. 1989. Optimizing soil and fertilizer nitrogen use by intensively managed winter wheat. 1. Crop nitrogen uptake. Agron. J. 81:116-120. Baethgen, W. E., and M. M. Alley. 1989. Optimum soil and fertilizer nitrogen use by intensively managed winter wheat. II . Critical levels and optimum rates of nitrogen fertilizer. Agron. J. 81: 120-125. Batey, T. 1977. Prediction by leaf analysis of nitrogen fertilizer required for winter wheat. J. Sci. Food Agric. 28: 275-278. Bauer, A., A. B. Frank, and A. L. Black. 1984. Estimation of spring wheat leaf growth rates and anthesis from air temperature. Agron. J. 76: 829-835. Bauer, A., A. B. Frank, and A. L. Black. 1987. Aerial parts of hard red spring wheat. II. Nitrogen and phosphorus concentration and content by plant development stages. Agron. J. 79:852-858. Beauchamp, E. G., W. D. Reynolds., D. Brasche-Villeneuve, and K. Kirby. 1986. Nitrogen mineralization kinetics with different soil pretreatment and management histories. Soil Sci. Soc. Am. J. 50:1478-1483. Becker, F. A., and W. Aufhammer. 1982. Nitrogen fertilization and methods of predicting the N requirements of winter wheat in Federal Republic of Germany. Proc. Fert. Soc. 211:33-66. 114 Beuerlein, J. E., E. S. Op linger, and D. Reicosky. 1992. Mineral content of soft winter wheat as influenced by nitrogen fertilization and management. Commun. Soil Sci. Plant Anal. 23:455-467. Binkley, D., and S. C. Hart. 1989. The components of nitrogen availability assessment in forest soils. Adv. Soil Sci. 10:57-112. Blade, S. F., and R. J. Baker. 1991. Kernel weight response to source-sink changes in spring wheat. Crop Sci. 31:1117-1120. Bock, B. R. 1984. Efficient use of nitrogen in cropping systems. p. 273-294. In R.D. Hauck et al. (ed.) Nitrogen in crop production. ASA, CSSA, and SSSA, Madison, WI. Bock, B. R., and K. R. Kelly. 1992. Predicting N fertilizer needs for corn in humid regions. TVA Bull. Y-226. Natl. Fert. And Environ. Res. Ctr. Muscle Shoals, AL. Boquest, D. J., and C. C. Johnson. 1987. Fertilizer effect on yield, grain composition, and foliar disease of double crop soft winter wheat. Agron. J. 79:135-141. Breland, T. A. 1994. Enhanced mineralization and denitrification as a result of heterogeneous distribution of clover residue. Plant Soil. 166:1-12. Bremner, J. M. 1965. Nitrogen availability indexes. In C.A. Black et al. (ed.) Methods of soil analysis, part 2. Agronomy. 9:1149-1176. Broadbent, F. E., and A. B. Carlton. 1978. Field trials with isotopically labeled nitrogen fertilizer. p. 1-14. In D. R. Nielsen and J. G MacDonald (ed.) Nitrogen in environment. Vol. 1. Nitrogen behavior in field soil. Academic Press, New York. Bundy, L. G., and E. S. Malone. 1988. Effect of residual profile nitrate on corn response to applied nitrogen. Soil Sci. Soc. Am. J. 52:1377-1383. Bundy, L. G., M. A. Schmitt, and G. W. Randall. 1992. Predicting N fertilizer need for corn in humid regions: Advances in the upper Midwest. p. 73-89. In B.R. Bock and K. R. Kelly (ed.) Predicting N fertilizer need for corn in humid regions. Bull. Y-226 Natl. Fertilizer and Environ. Res. Muscle Shoals, AL. Burnett, C. A. 1975. The agriculture use of organic material in Brazil. FAO Soil Bulletin. 27:305-311. Buxton, D.R., and G.C. Marten. 1989. Forage quality of plant parts of perennial grasses and relationship to phenology. Crop Sci. 29:429-435. 115 Cabrera, M. L., and D. E. Kissel. 1988. Evaluation of a method to predict nitrogen mineralized from soil organic matter under field conditions. Soil Sci. Soc. Am. J. 52: 1027-1031. Campbell, C. A., Y. W. Jame, and R. D. Jong. 1988. Predicting net nitrogen mineralization over a growing season: Model verification. Can. Soil Sci. 68:537­ 552. Cassman, K. G., and D. N. Munns. 1980. Nitrogen mineralization as affected by moisture, temperature and depth. Soil Sci. Soc. Am. J. 44:1233-1237. Cochran, V. L., R. L. Warner, and R. I. Papendick. 1978. Effect of N Depth and application rate on yield, protein content, and quality of winter wheat. Agron. J. 70:964-968. Collins, H. P., P. E. Rasumussen, and C.L. Douglas, Jr. 1992. Crop rotation and residue management effects on soil carbon and microbial dynamics. Soil Sci. Soc. Am. J. 56:783-788. Cook, R. J. 1988. Biological control and holistic plant-health care in agriculture. Am. J. Alt. Agri. 3:51-62. Copeland, P. J., and R. K. Crookston. 1992. Crop sequence affects nutrient composition of corn and soybean growth under high fertility. Agron. J. 84:503-509. Costa, J. M., and W. E. Kronstad. 1994. Association of grain protein concentration and selected traits in hard red winter wheat population in the Pacific Northwest. Crop Sci. 34:1234-1239. Cox, F. 1985. Survey of state soil testing laboratories in the United States. Soil Sci. Soc. Am. J. Madison, W I. Dahnke, W. C., and G. V. Johnson. 1990. Testing soils for available nitrogen. p. 127-139. In R. L. Westerman (ed.) Soil testing and plant analysis. ASA, CSSA, and SSSA, Madison. WI Dalal, R. C. 1989. Long-term effect of no-tillage, crop residue, and nitrogen application on properties of a vertisol. Soil. Sci. Soc. Am. J. 53:1511-1515. Desai, R.M., and C.R. Bathia. 1978. Nitrogen uptake and nitrogen harvest index in durum wheat. Euphytica. 27:561-566. 116 Doll, E. C. 1962.Effect of fall-applied nitrogen fertilizer and winter rainfall on yield of wheat. Agron. J. 54:471-473. Donohue, S. J., and D. E. Brann. 1984. Optimum N concentration in wheat grown in the coastal plan region of Virginia. Commun. Soil Sci. Plant Anal. 15: 651-611. Doran J. W., and J. F. Power. 1983. Effect of tillage on the nitrogen cycling in corn and wheat production. In nutrient cycling in agriculture ecosystem. University of Georgia. Athens. Special publication No. 23:441-455. Echeverria, H. E., C. A. Navarro, and F. H. Andrade. 1992. Nitrogen nutrition of wheat following different crops. J. Agric. Sci. 118:157-163. Ellert, B. H., and J. R. Bettany. 1992. Temperature dependence of net nitrogen and sulfur mineralization. Soil Sci. Soc. Am. J. 65:1133-1141. Engel, R. E., and J. C. Zubriski. 1982. Nitrogen Concentration in spring wheat atseveral growth stages. Commun. Soil Sci. Plant Anal. 13:531-534. Eno, C. F. 1960. Nitrogen production in the field by incubating the soil in polyethylene bags. Soil Sci. Soc. Am. Proc. 47:277-279. Federer, C. A. 1983. Nitrogen mineralization and nitrification: Depth variation in four New England forest soils. Soil Sci. Am. J. 47:1008-1014. Fiez, T. E., W. L. Pan, and B. C. Miller. 1995. Nitrogen use efficiency of winter wheat among landscape positions. Soil Sci. Am. J. 59: 1666-1671. Foth, D. H., and B. G. Ellis. 1988. The soil nitrogen cycle. In Soil Fertility. John Wiley & Sons. Ins. p. 69-73. Fox, R. H., and W. P. Piekielek. 1978. Field testing of several nitrogen availability indices. Soil Sci. Soc. Am. J. 42:747-750. Fox, R. H., and W. P. Piekielek. 1984. Relationship between anaerobically mineralized nitrogen, chemical indexes, and nitrogen availability to corn. Soil Sci. Soc. Soc. Am. J. 48:1087-1090. Franzluebbers, A. J., F. M. Hons, and D. A. Zuberer. 1994. Long-term changes in soil carbon and nitrogen pools in wheat management systems. Soil Sci. Soc. Am. J. 58:1639-1645 117 Fyson, A., and Oaks, A. 1990. Growth propotion of maize by legume soils. Plant Soil. 122:259-266. Gelderman, R. H., W. C. Dahnke, and L. Swenson. 1988. Correlation of several N indices for wheat. Commun. Soil Sci. Plant Anal. 19(6):755-772 Gauer, L. E., C. A. Grant, D. T. Gehl, and L. D. Bailley. 1992. Effects of nitrogen fertilizer on grain protein content, nitrogen uptake, and nitrogen use efficiency of six spring wheat cultivars, in relation to estimated moisture supply. Can. J. Plant Sci. 72: 235-241. Gelderman, R. H., W. C. Dahnke, and L. Swenson. 1988. Correlation of several N indices for wheat. Commun. Soil Sci. Plant Anal. 19 (6):755-772. Gianello, C., and J. M. Bremner. 1986. Comparison of chemical methods of assessing potentially available organic nitrogen in soil. Commun. Soil Sci. Plant Ana. 17:215-236. Goos, R. J., and L. Prunty. 1990. Yield variability and the yield goal decision. In J. L. Havlin (ed.) Proc. Of Great Plain Soil Fertility Conf., Denver, CO. 6-7 Kansas State Univ. Manhattan. Gupta, V. R., and J. J. Germinde, 1988. Distribution of microbial biomass and its activity in different soil aggregate size classes as affected by cultivation. Soil Biol. Biochem. 17:517-523. Hadas, A., S. Feigenbaum., A. Feigin., and R. Portnoy. 1986. Nitrogen mineralization rate in profile of differently managed soil types. Soil Sci. Am. J. 50: 314-319 Harper, J. E. 1984. Uptake of Organic Nitrogen Forms by Roots and Leaves. In R. D. Hauck(ed.) Nitrogen In Crop Production. Am. Soc. Agro. Madison, WI. Harper, L.A., R.R. Sharp, G.W. Langdale, and J.E.Giddens. 1987. Nitrogen cycling in wheat crop: Soil, plant and aerial nitrogen transport. Agron. J. 79:965-973. Hassink, J. 1994. Active organic fractions and microbial biomass as predictors of N mineralization. Eur. J. Agron. 3:257-26. Holmes, W. E. 1994. Soil microbial biomass dynamics and net mineralization in northern hardwood ecosystems. Soil. Sci. Soc. Am. J. 58:238-243. 118 Houot, S., J. A. E. Molina, R. Chaussod, and C.E. Clapp. 1989. Simulation by NCSSOIL of net mineralization in soil from the Deherain and 36 Parcelles fields at Grignon. Soil Sci. Am. J. 53:451-455. Jansson, S. L. 1971. Use of 15N in studies of soil nitrogen. Soil Biol. Biochm. 2:129-166. Jarvis, S. C., A. S Elizabeth., M. A. Shepherd, and D. S. Powlson,. 1996. Nitrogen mineralization in temperate agriculture soils: Processes and measurement. Advances in Agronomy. Vol. 57. Academic Press. pp 187-230. Johnston, A. M., and D. B. Fowler. 1991. No-till winter production: response to spring applied N fertilizer form and placement. Agron. J. 83: 722-728. Karrou, M., and J. W. Maranville. 1994. Response of wheat cultivars to different soil nitrogen and moisture regimes: II. Nitrogen uptake, partitioning and influx. J. Plant Nut. 17: 745-761. Keeney, D. R. 1982. Nitrogen availability indices. p 711-733. In A.L. Page et al. (Ed.) Methods of Soil Analysis. Part 2. Agron. Monrog. ASA and SSSA, Madison, WI. Keeney, D. R., and D. W. Nelson. 1982. Nitrogen inorganic forms. p. 643-698. In A. L. Page et al. (ed.) Methods of soil analysis. Part 2. 2nd ed. Agron. Monogr. 9. ASA ans SSSA, Madison, Wl. Kelly, K.W. 1995. Rate and timing of nitrogen application for wheat following different crops. J. Prod. Agric. 8:339-345. Klepper, B., B.R. Rickman, and C.M. Peterson. 1982. Quantitative characterization of vegetative development in small grain cereals. Agron. J. 74:789-792. Lal, P., G. G. Reddy, and M. S. Modi. 1978. Accumulation and redistribution pattern of dry matter and N in triticale and wheat varieties under water stress conditions. Agron. J. 70: 623-626. Large, E. C. 1954. Growth stages in cereals. Illustration of the Feekes scale. Plant Pathol. 3:128-129. Liang, B. C., M. Remillard, and A. F. MacKenzie. 1991. Influence of fertilizer, irrigation, and non-growing season precipitation on soil nitrate-nitrogen under corn. J. Environ. Qual. 20:123-128. Linn, D. M., and J. W. Doran. 1984. Effect of waterfilled pore and space on CO2 and NO2 production in tilled and no-tilled soils. Soil Sci. Soc. Am. J. 48:1267-1272. 119 Loiseau, P., R. Chaussod, and R. Delpy. 1994. Soil microbial biomass and in situ nitrogen mineralization after 20 years of different nitrogen fertilization and forage cropping system. Eur. J. Agron. 3:327-332. Lund, V., and B. Goksoyr. 1980. Effects of water fluctuations on microbial mass and activities. Micro. Eco. 6:115-123. Luxova, M. 1988. The participation of the primary maize root in the assimiluation of NH4-N ions. Plant Soil. 111:187-189 Magdoff, F. R. 1991. Understanding the Magdoff pre-sidedress nitrate test for corn. Prod. Agric. 4:297-305. Maples. R., J. G. Keogh, and W.E. Sabbe. 1977. Nitrate monitoring in cotton production in Loring- Calloway silt loam. Arkansas Agric. Exp. Stn. Bull. No. 825. Marumoto, T., J. P. Anderson, and K.H. Domsch.1982. Mineralization of nutrients from soil microbial biomass. Soil Bio. Biochem. 14: 469-475. Mary, B., and S. Recous. 1994. Measurement of nitrogen mineralization and mobilization fluxes in soil as a mean of predicting net mineralization. Eur. J. Agron. 3:291-300. McDonald, G.K. 1992. Effects of nitrogenous fertilizer on growth, grain yield and grain protein concentration of wheat. Aust. J. Agric. Res. 43: 949-967. McKenney, D. J., S. W. Wang., C. F. Drury, and W. I. Findlay. 1995. Denitrification, immobilization, and mineralization in nitrate limited and non limited residueamended soil. Soil Sci. Soc. Am. J. 59:118-124. McNeal, F. H., M. A. Berg, and C. A. Watson. 1968. Nitrogen and dry matter in five spring varieties at successive stages of development. Agron. J. 58:605-608. McTaggert, I. P., and K. A. Smith. 1993. Estimation of potentially mineralizable nitrogen in soils by KC1 extraction: Comparison with plant uptake in the field. Plant Soil. 157-184. Meisinger, J. J., R. F. Mulford, and J. S. Stangarone. 1987. Effect of residual N on winter wheat in Maryland. p. 209. In: Agronomy Abstracts. ASA, Madison, Wl. Memon, G. H. and G. H. Jamro. 1988. Influence of nitrogen fertilization on grain protein content and NPK content of straw in late sown wheat. Pak. J. Sci. Ind. Res 31:649-650. 120 Michrina, B. P., R. H. Fox, and W. P. Piekielek. 1981. A comparison of laboratory, greenhouse and indicates of nitrogen availability. Commun. Soil Sci. Plant Anal. 12:519-535. Moorby, J., and R. T. Besford. 1983. Mineral nutrition and growth. p. 481-527. In A. Lauchli and R.L. Bieleski (ed) Encyclopedia of plant plant physiology. Vol. 5B. Springer-Verlag. Berlin. Neve, S.D., J. Pannier, and G. Hofman. 1994. Fraction of vegetable crop residues in relation to in situ N mineralization. Eur. J. Agron. 3:267-272. Neve, S. D., J. Pannier, and G. Hofman. 1994. Fraction of vegetable crop residues in relation to insitu N mineralization. Eur. J. Agron. 3:267-272. Nielsen, D. C., and A. D. Halvorson. 1991. N fertility influence on water stress and yield of winter wheat. Agron. J. 83:1066-1070. Olson, R.A., and L.T. Kurtz. 1982. Crop N requirements, utilization and fertilization. p. 567-604. In F.J. Stevenson (ed.) Nitrogen in agricultural soils. Agron. Monogr. 22. ASA, CSSA, and SSSA, Madison, WI. Orchard, V. A., and Cook, F. J. 1988. Relationship between soil respiration and soil moisture. Soil Biol. Biochem. 15:447-453. Ower, J. C. 1980. Nitrate leaching as affected by artificial drains. M.S. Thesis Oregon State University, Corvallis, OR Palm, C. A., and P. A. Sanchez. 1991. Nitrogen release from some tropical legumes as affected by lignin contents. Soil Biol. Biochem. 23:83-88. Parnas, H. 1975. Model for decomposition of organic material by microorganisms. Soil Biol. Bioch. 7:161-169. Papastylianou, I., R. D. Graham, and D. W. Puckridge. 1984. Diagnosis of the nitrogen status of wheat at tillering and prognosis for maximum grain yield. Commun. Soil Agron. 22:651-688. Peter C. S., M. M Alley, and Y.Z. Lei. 1993. Spring nitrogen on winter wheat: I. Farmerfield validation of tissue test-based rate recommendations. Agron. J. 85:1181­ 1186. Power, J. F., and J. W. Doran. 1984. Nitrogen Use in Organic Farming. In R. D. Hauck(ed.) Nitrogen in Crop Production. ASA. Madison, WI. 121 Power, J. F., and J. Alessi. 1978. Tiller development and yield of standard and semidwarf spring wheat varieties as affected by nitrogen fertilizer. J. Agric. Sci. 90:97-108 Qian. P., J. J. Schoenau, and A. Braul. 1993. Assessing mineralizable soil organic N and S using anion exchange. p. 257, in Agronomy Abstracts. ASA, Madison, Wl. Rauschkolb, R. S., T. L. Jackson, and A. I. Dow. 1984. Management of nitrogen in the pacific States. p. 765-777. In R.D. Hauck (ed.) Nitrogen in Crop Production. ASA. Madison, WI. Recous, S., J. M. Machet, and B. Mary. 1988. The fate of 15 N urea and ammonium nitrate applied to a winter wheat crop. Plant Science. 122: 215-224. Rees, R. M., I. P. McTaggart., K.A. Smith, and E. A. Stockdale. 1994. Methodology for the study of N mineralization in the field. Eur. J. Agron. 3: 301-309. Rice, C. W., and, J. L Havlin. 1994. Integrating mineralizable nitrogen indices into fertilizer nitrogen recommendations. p. 1-13. In Soil testing: Prospects for improving nutrient recommendations. SSSA Special publication number 40. Madison WI. Robinson, F. E., D. W. Cudney, and W. L. Lehman. 1979. Nitrogen fertilizer timing, irrigation, protein, and yellow berry in durum whaet. Agron. J. 71:304-307. Roth, G. W., and R. H. Fox. 1990. Soil nitrate accumulations following nitrogen-fertilized corn in Pennsylvania. J. Envion. Qual. 19:243-248. Roth, G. W. 1987: Evaluation of plant tissue and soil test for predicating nitrogen fertilizer requirements of winter wheat. Ph.D Thesis. Pennsylvania State Univ. University Park, PA. Roth, G. W., R. H. Fox, and H .G. Marshall. 1989. Plant tissue test for predicting nitrogen requirements of winter wheat. Agron. J. 81:502-507. Scharf, P. 1993. Development of field-specific spring N recommendations for winter wheat. Ph. D. Thesis. Virginia polytechnic Institute abs State Univ. Blacksburg, Virginia. Sarrantonio, M., and T. W. Scott. 1988. Tillage effects on nitrogen availalility to corn following a winter green manure crop. Soil Sci. Soc. Am. J. 52:1661-1668. Seneviratne, R., and A. Wild. 1984. Effect of mild drying on the mineralization of soil nitrogen. Plant and Soil. 84:175-179. 122 Schepers, J. S., K. D. Frank, and C. Bourg. 1986. Effect of yield goal and residual soil nitrogen concentration on N fertilizer recommendations for irrigated maize in Nebraska. J. Fert. Issues. 3:133-139. Schmitt, M. A., G. W. Randall, G. W. Rehm, and G. L. Malzer. 1991. Calibration and correlation of soil N test for corn's N recommendations. p. 212-221. In A report on field research in soils. Minn. Agric. Exp. Stn. Misc. Publ. 71. Sebastain, K. D. 1995. Nitrogen fertilization of winter wheat grown in four rotations in western region. M.S. Thesis. 101. pp. Oregon State University, Corvallis, OR. Serna, M. D., and F. Pomares. 1992. Evaluation of chemical indices of soil organic nitrogen availability in calcareous soil. Soil Sci. Soc. Am. J. 65:1486-11491. Smith J. L., R. I. Papendick., D. F. Besdicek, and L. M. Lynch. 1993. Soil organic matter dynamics and crop residue management. In Soil applications in agricultural and environmental management. (F.B. Metting Jr., Ed), pp. 65-94. Microbial Ecology. Marcel Dekker Inc, New York. Smith, J. L. 1994. Cycling of nitrogen through microbial activity. In advances in soil Science. Lewis publishers. Pp 91-119. Sparling, G. P., and D. J. Ross. 1988. Microbial contributions to the increased nitrogen mineralization after air-drying of soils. Plant Soil. 105:163-167 Stanford, G., and J.O. Legg. 1984. Nitrogen and yield potential. In R.D. Hauck (ed.) Nitrogen in crop production. ASA, Madison, WI. p 263-293. Stanford, G. 1982. Assessment of soil N availability. p. 651-688. In F. J. Stevenson (ed). Nitrogen in Agriculture Soils. Agron. monogr. 22. ASA. CSSA, and SSSA, Madison, WI. Stanford, G., and E. Epstein. 1974. Nitrogen mineralization-water relations in the soils. Soil Sci. Soc. Am. Proc. 38:103-107. Stanford, G., and S. J. Smith. 1972. Nitrogen Mineralization potentials of Soils. Soil Sci. Soc. Amer. Proc. 36:465-472. Tennant, D. 1976. Root growth of wheat. I. Early patterns of multiplication and extension of wheat roots including effects of level of N, P, and K. Aust. J. Agric. Res. 27:183-96. 123 Thicke, F. E., M. P. Russel le., 0. B. Hesterman, and C. C. Sheaffer, 1993. Soil nitrogen mineralization indexes and corn response in crop rotation. Soil Science. 156:322­ 334. Tisdale, S. L., W. L. Nelson, and J. D. Beaton. 1985. Soil fertility and fertilizers. New Yark. Macmillan Publishing Company. Vanotti, M. B., and L. G. Bundy. 1994. Frequency of nitrogen fertilizer carryover in the humid midwest. Agron. J. 86:881-886. Vaughan, B., K. A. Barbarick., D. G. Westfall, and P. L. Chapman. 1990. Tissue nitrogen levels for dryland hard red winter wheat. Agron. J. 82:561-565. Vaughan, B., D.G. Westfall, and K.A. Barbarick. 1990. Nitrogen rate and timing effects on winter wheat grain yield, grain protein, and economics. J. Prod. Agric. 3:324­ 328. Waldren, R.P., and A.D. Flowerday. 1979. Growth stages and distribution of dry matter, N, P, and K in winter wheat. Agron . J. 71:391-397. Wang, J. G., and R. L. Bakken. 1989. Nitrogen mineralization in the rhizosphere and non­ rhizoshere soil, effect of spatial distribution of N-rich and N-poor plant residue. In: J.A. Hansen. and K. Henriksen (eds.) Nitrogen in organic wastes applied to soils. London: Academic Press, pp 81-97. Wani, S. P., W. B. McGill, and L. A. Robertson. 1991. Soil N dynamics and N yield of barley grown on Breton loam using biological fixation or fertilizer. Bio. Ferti. Soils. 12:1018. Wehrmann, J., and H. C. Scharf. 1979. De mineralstickstoffgehalt des bodens als mabstab fur den stickstoffdungerbedarf . Plant Soil. 52:109 West, C. P., D. W. Walker, R.K. Bacon, D.E. Longer, and K.E. Turner. 1991. Phonological analysis of forage yield and quality in winter wheat. Agron. J. 83:217-224. Westfall, D. G. 1984. Management of nitrogen in the Mountain states. p. 751-763. In R.D. Hauck (ed.) Nitrogen in crop production. ASA. Madison, WI. Whitmore, A. P., and J. R. Groot. 1994. The mineralization of N from finely or coarsely chopped crop residues: measurement and modeling. Eur. J. Agron. 3:367-373. 124 Wilson, C. E., R. J. Norman, and B. R. Wells. 1994. Chemical estimation of nitrogen mineralization in paddy rice soils: I. Comparison to laboratory indices. Commun. Soil Sci. Plat Anal. 25:573-590. Xingting, W., and F. E. Below. 1992. Root growth, nitrogen uptake, and tillering of wheat induced by mixed-nitrogen source. Crop Sci. 32: 997-1002. 125 APPENDIX Appendix Table 1. Agronomic data of wheat following clover for 1994-95 growing season (Helle Ruddenklan site) Maturity Feekes 5 9-m samples Treatment Kg ha' DM N cont Plant he 1000 Sw9 Tw Kg ha' (%) (cm) (g) (kg ili3) ( %) Plot Yield Grain chaff Straw Protein DM N cont. DM N Yield N Kg ha % Kg ha' % Kg ha' % Kg ha' 0 524 4.29 76 58.2 529 8.02 7666 0.29 1228 0.59 6194 1.38 5007 56 598 4.28 84 59.0 548 8.18 10247 0.39 1602 0.53 7325 1.37 6163 112 676 4.48 82 58.4 503 8.35 9782 0.39 1784 0.67 8376 1.60 6028 168 676 4.05 88 58.2 558 8.77 12436 0.49 2124 0.86 9581 1.62 6756 0 824 3.98 76 57.8 515 8.25 11086 0.30 1003 0.42 4837 1.37 5033 56 674 3.96 85 57.9 498 8.23 9283 0.36 1612 0.61 7510 1.43 5793 112 588 4.37 86 57.4 575 8.66 10503 0.41 1831 0.50 8001 1.49 6226 168 567 4.04 86 57.0 554 8.69 11587 0.33 2073 0.79 9413 1.58 6114 0 507 4.31 74 57.5 537 7.96 7518 0.35 1200 0.54 5546 1.33 4812 56 493 3.98 84 57.5 557 8.42 9002 0.36 1479 0.74 6912 1.36 5239 112 532 3.90 85 58.0 576 8.44 10050 0.51 1715 0.78 8271 1.44 6162 168 750 4.23 87 57.7 544 9.02 11512 0.43 1833 0.71 8622 1.70 5905 Plant height, 1111Seed weight, Test weight Appendix Table 2. Agronomic data of wheat following clover for 1995-96 growing season (Helle Ruddenklan site) Maturity Feekes 5 Treatment Kg ha' DM Kg ha' N (%) Plant htl (cm) 1000 Swill T«w§ (g) (kg m-3) (%) Grain Straw Protein DM N cont. Yield N cont. Kg ha' % Kg ha' % Yield Kg ha' 0 737 3.99 80 55.2 746 7.73 5582 0.22 4745 1.24 4858 56 620 4.05 88 56.0 744 7.34 7751 0.20 6314 1.19 6161 112 710 3.61 88 58.4 749 8.44 8951 0.34 7801 1.73 7979 168 694 3.76 88 58.2 759 8.91 9026 0.31 7526 1.43 8905 224 741 4.16 91 56.4 757 10.24 11033 0.45 8295 1.81 9060 0 635 4.30 82 13: 746 7.65 5388 0.21 4676 1.28 4765 56 745 4.00 88 57.4 748 7.86 7045 0.21 5607 1.31 6680 112 631 4.01 93 57.0 746 8.27 10489 0.32 6901 1.34 7990 168 545 4.45 93 56.6 760 9.08 8120 0.44 7395 1.57 8654 224 502 4.00 93 57.5 767 9.96 10727 0.50 8676 1.84 9535 0 561 4.62 80 gN 747 7.69 5063 0.19 4876 1.30 4469 56 643 4.46 88 57.7 745 7.84 11414 0.22 6864 1.28 6715 112 467 4.42 96 56.3 753 8.52 11496 0.29 8283 1.41 7993 168 459 4.48 91 58.1 760 9.22 10589 0.36 8045 1.52 8823 224 737 4 25 93 54.3 752 9 91 13433 0 61 9977 1 99 8653 ¶ Plant height, 7 Seed weight, § Test weight Appendix Table 3. Agronomic data of wheat following corn for 1994-95 growing season (Volker site) Maturity Feekes 5 9-m samples Treatment Kg ha' DM N cont Plant he 1000 swn Tw* Kg ha' (%) (cm) (g) (kg ni3) Protein (%) Plot Yield Grain chaff Straw DM N cont. DM N Yield N Kg ha % Kg ha' % Kg ha' % Kg ha' 0 951 4.38 76.7 45.7 725 7.53 12634 0.36 1190 0.63 4872 1.26 4199 56 577 4.21 86.6 44.1 735 7.47 13146 0.34 1756 0.41 6645 1.33 6261 112 833 3.46 90.2 41.9 738 8.11 14130 0.42 2023 0.8 8622 1.42 7150 168 879 4.56 90.7 38.3 744 9.23 17067 0.57 1061 1.11 10168 1.79 7970 0 682 5.50 74.4 44.8 737 7.86 6313 0.29 1537 0.53 6626 1.21 5367 56 708 4.19 87.1 41.6 735 8.10 10526 0.37 2112 0.45 8314 1.27 6611 112 689 4.24 92.2 39.2 734 8.99 13693 0.43 2101 0.73 9593 1.69 7578 168 485 3.53 90.9 41.0 749 9.99 14795 0.65 2193 0.76 9470 1.73 7707 0 761 3.97 79.5 44.3 728 8.71 9387 0.28 1222 0.68 5681 1.33 6056 56 833 5.17 86.9 44.5 745 8.37 12897 0.38 1915 0.57 6626 1.41 6013 112 551 4.34 92.7 40.0 747 9.14 15934 0.48 2379 0.77 9785 1.67 7920 93.0 3.37 833 168 Plant height, uu Seed weight, § Test weight 39.7 752 10.22 16643 0.7 2805 0.59 11187 1.78 8194 Appendix Table 4. Agronomic data of wheat following corn for 1995-96 growing season (Volker site) Maturity Feekes 5 Treatment Kg ha-1 DM Kg ha' N ( %) Plant htll (cm) 1000 Sw" TIO (g) (kg ni3) (%) Grain Straw Protein DM N cont. Yield Kg ha-1 % Kg ha-1 Yield N cont. Kg ha' 0 302 4.99 80 50.7 753 6.4 6060 0.20 3760 1.10 3932 56 411 5.02 88 50.1 753 6.7 12295 0.23 6848 1.15 5642 112 363 5.03 90 51.2 760 7.6 15413 0.33 7790 1.41 8133 168 332 4.93 92 52.1 756 7.7 18859 0.42 7265 1.51 7811 224 341 5.39 96 51.4 769 9.0 17552 0.53 8681 1.87 7748 0 341 5.09 82 52.1 763 7.0 8900 0.22 4892 1.16 4858 56 428 5.24 88 51.1 756 7.0 11857 0.20 6440 1.21 6854 112 319 4.64 93 51.2 765 7.5 14975 0.25 7608 1.31 7503 168 315 5.45 96 52.1 772 8.1 13974 0.40 7214 1.51 8280 224 367 4.04 97 52.4 772 9.1 15208 0.63 7287 1.86 8505 0 428 5.23 80 55.7 758 6.8 9170 0.18 5089 1.11 4889 56 389 4.88 88 50.1 761 7.0 15274 0.20 5279 1.16 6039 112 354 4.78 96 51.2 771 8.1 15632 0.31 7498 1.39 8056 168 363 5.05 98 52.1 772 8.2 16179 0.49 6359 1.52 8242 98 5.11 Plant height, 111 Seed weight, § Test weight 51.4 774 9.0 14668 0.51 7097 1.82 8502 224 385 Appendix Table 5. Agronomic data of wheat following grass for 1994-95 growing season (Van Leeuwen site) Maturity Feekes 5 9-m samples Treatment Kg ha-1 DM Kg ha' N cont Plant he 1000 SwIr Tw Protein (%) (cm) (g) (kg in-3) (%) Plot Yield Grain chaff Straw DM N cont. DM N Yield N Kg ha % Kg ha' % Kg ha' % Kg ha' 0 735 3.14 71 56.8 483 8.52 7078 0.24 960 0.66 3967 1.43 3180 67 604 4.06 87 57.8 548 9.19 12372 0.45 1817 0.70 7240 1.44 4989 134 649 3.94 87 56.8 470 9.21 18121 0.66 2733 0.84 11574 1.69 5298 201 846 3.40 93 57.2 470 10.04 14786 0.51 2506 0.65 10870 1.70 5978 0 407 4.24 71 56.9 535 8.63 7448 0.43 1102 0.70 4614 1.43 3184 67 577 3.82 86 57.7 535 8.34 12446 0.43 1745 0.67 7522 1.38 5279 134 584 4.31 87 57.0 509 9.35 13319 0.62 1979 0.69 7028 1.69 5090 201 663 3.76 91 57.0 464 9.95 17150 0.65 2034 0.70 8698 1.63 5903 0 525 4.36 76 57.9 580 8.68 8060 0.32 1176 0.60 4824 1.32 3211 67 551 3.91 85 58.1 567 8.21 11316 0.49 1563 0.62 6341 1.42 6231 134 781 3.88 87 58.1 509 8.95 12882 0.62 1835 0.57 7544 1.58 7173 201 590 3.97 94 57.9 535 9.90 14537 0.64 2184 0.54 8928 1.75 6984 ¶ Plant height, 1111 Seed weight, § Test weight Appendix Table 6. Agronomic data of wheat following grass for 1995-96 growing season (Chipman site) Maturity Feekes 5 Treatment Kg ha-1 DM Kg ha-1 N (%) Plant he (cm) 1000 swn (g) TNO (kg in") (%) Grain Straw Protein DM N cont. Yield N cont. Kg hal °A Kg ha-1 °A Yield Kg ha-1 0 907 3.79 87 745 8.7 9689 0.29 6564 1.45 4876 56 870 3.87 87 743 9,1 12946 0.32 8445 1.59 6140 112 1061 4.23 87 751 10.1 8658 0.56 7482 2.00 7285 168 1222 3.98 93 741 11.4 13640 0.59 7545 2.21 6322 224 1140 3.60 96 735 12.2 16053 1.11 6182 2.45 5763 0 1084 3.64 86 741 8.8 8345 0.27 5013 1.47 4212 56 1114 3.98 87 738 9.3 12039 0.31 6514 1.61 5445 112 1091 3.53 91 728 10.3 13990 0.53 7895 2.00 5620 168 971 3.44 95 730 11.0 13296 0.71 6426 2.24 5804 224 1234 3.72 95 702 12.4 16878 1.08 7995 2.51 4914 0 930 2.89 84 723 9.1 5995 0.31 4657 1.45 3874 56 979 3.66 87 731 9.1 11896 0.40 7132 1.64 4812 112 919 3.93 92 722 9.7 10789 0.53 6170 1.70 5209 168 821 3.16 98 684 11.2 10989 0.54 5482 2.05 4879 98 2 25 900 224 Plant height, 111 Seed weight, § Test weight 688 11.7 13658 0 81 7889 2 23 5238 132 Appendix Table 7. Soil test results through winter months of all on-farm sites for 1994-95 growing season. Mineralizable N Date of Soil depth NO3-N NH4-N sampling cm mg kg-1 mg kg-1 mg kg-1 Clover-wheat rotation 1-05-95 2-25-95 0-30 30-60 60-90 36-48 2.7 2.9 0-30 30-60 60-90 36-48 5.5 3.4 3.5 3.7 3.5 1.6 4.5 11.6 10.1 9.6 5.6 0.4 1.9 11.8 9.1 3.7 2.1 2.1 2.3 16.1 4.9 2.4 3.3 19.8 6.5 1.9 2.6 Corn-wheat rotation 11-1-94 0-30 30-60 60-90 3.6 2.5 3.8 12-22-94 0-30 30-60 60-90 36-48 4.2 2.8 0-30 30-60 60-90 5.2 5.0 0-30 30-60 60-90 36-48 1-23-95 2-7-95 1.4 2.4 4.0 3.0 5.4 1.1 12.1 1.2 0.8 5.5 3.5 4.8 5.6 3.3 2.8 1.5 1.2 1.1 1.3 14.6 8.2 0-30 30-60 60-90 16.2 5.8 17.5 3.0 3.0 4.9 5.9 0-30 30-60 60-90 36-48 3.4 9.0 1.6 3.1 7.1 0-30 30-60 60-90 36-48 4.5 3.8 6.0 3.0 4.9 3.3 Grass-wheat rotation 11-1-94 12-22-94 2-6-95 2.4 1.8 23.7 5.7 6.7 5.6 1.6 1.7 25.1 7.5 133 Appendix Table 8. End of season soil test results of clover-wheat rotation for 1994-95 growing season. NO3-N Mineralizable N Block Treatment NH4 -N Depth mg kg' mg kg-' mg kg-1 1 1 3.4 1 1 2 3.5 1 1 3 2.8 1 2 1 2 2 2 3 2.1 1.6 1.2 1 3 1 1.8 1 3 2 0.8 1 3 3 1 1 2 1 4 4 4 2 1 1 1 1 2 2 1 3 1 2 1 3 2 2 2 1 2 2 2 2 3 2 2 2 2 2 2 3 1 3 2 3 3 4 4 4 2 3 1 1 3 1 2 3 1 3 3 2 3 2 2 2 3 3 1 3 3 2 3 3 3 3 4 4 4 2 3 3 3 Treatments Depth 1 1 1 = 0 kg N hi 1 3 1 3 1 3 2 = 56 kg N hi = 30 cm 2= 60 cm 3= 90 cm 1.3 4.3 2.9 4.1 4.1 3.2 2.6 4.9 3.2 2.7 4.4 2.8 2.8 4.1 3.3 3.2 4.0 3.6 3.0 4.4 4.0 3.8 3.9 3.9 2.9 3.8 2.9 2.6 3 4.6 0.4 0.4 3.6 0.4 0.4 4.4 0.4 0.4 4.2 0.4 0.4 4.7 0.5 0.4 4.3 0.4 0.4 4.9 0.4 0.4 4.4 0.4 0.4 3.6 0.6 0.4 5.1 0.5 27.3 8.6 23.7 10.4 29.8 9.2 25.7 9.3 29.5 14.6 29.1 11.4 32.2 9.5 34.1 12.6 31.2 17.3 32.2 16.3 0.4 7.1 35.2 0.7 0.4 5.0 0.6 0.4 17.8 33.8 15.3 = 112 kg N hi 4 = 168 kg N h-1 134 Appendix Table 9. End of season soil test results of corn-wheat rotation for 1994-95 growing season. Treatment Block Depth NOrN Mineralizable N NH4 -N mg kg-1 mg kg-1 mg kg-1 17.0 10.2 1 1 1 1 2 2.7 2.0 3.9 1 1 1 3 2.1 1 1 2.4 2 1.8 1 2 2 2 3 1 3 1 1 3 2 1 3 3 1 4 4 4 2 5.8 3.8 2.7 2.1 3.2 2.2 2.6 2.2 2.4 0.6 3.7 0.9 0.4 3.3 1 1 1 2 2 2 2 2 2 2 2 2 2 2 2 1 1 2 1 3 1.7 7.6 2.2 2 2 2 2 3 1.6 1 4.3 2 2.0 3 3 3 1 3 1.5 4 4 4 2 2.5 5.9 3 1.8 1 1 2.2 1 2 1.7 1 3 2 2 2 3 3 3 3 Treatments Depth 3 1 3 3 3 3 3 3 3 3 3 3 1 1 1 2 3 1 2 1.5 2.0 2.6 1.5 2.2 2.2 2.3 2.3 1.0 15.5 9.4 26.7 10.0 1.2 0.5 3.1 24.7 1.1 15.8 0.5 3.1 1.0 19.8 14.6 0.4 3.4 1.0 0.5 3.3 1.0 0.4 3.9 17.2 12.4 21.4 14.4 24.7 1.2 16.2 0.4 2.2 0.4 0.4 22.1 13.2 1.5 19.9 0.7 0.4 18.1 1.0 1.0 22.6 12.7 0.4 25.1 4 0.4 1 16.9 4 0.9 2 2.1 4 3 2.1 0.4 1 = 0 kg N If' 2 = 56 kg N If' 3= 112 kg N WI 4= 168 kg N 1 = 30 cm 2= 60 cm 3= 90 cm 3 3 135 Appendix Table 10. End of season soil test results of grass-wheat rotation for 1994-95 growing season. Block Treatment Depth NO3-N Mineralizable N NH4-N mg kg' mg kg-1 mg kg-1 2.8 7.9 1.4 21.3 11.7 1 1 1 1 1 2 1 1 3 1.9 6.1 1 2 1 3.2 0.4 0.8 2 2 2 1.8 1.3 3 1 2.4 2.0 0.6 3 3 3 2 1.5 3 2.1 1 1 1 1 1 1.0 1.0 0.5 2.1 10.6 9.7 29.3 7.7 1 4 4 2 1 4 3 1 1 2.4 5.9 2.4 5.5 1 2 1.8 10.5 1.7 1 3 1 2.3 2.7 0.7 9.4 2 1.9 1.6 3 2.6 5.6 0.5 1 2 2 2 2 2 2 2 2 2 1 2 2 2 3 3 3 1 2 3 2 2 4 4 4 2 3 1 1 3 1 2 3 1 3 3 3 2 2 2 2 3 3 3 3 3 3 3 Treatments Depth 1 3 1.9 2.1 6.8 1.7 2 3.5 29.3 10.6 1.5 0.7 9 1.5 30.5 12.9 31.5 13.4 24.9 8.9 0.8 7 1.1 0.5 8.7 29.1 12.8 30.3 15.4 3 2.7 2.8 3.4 3.2 3.4 3 1 3.8 0.7 0.4 6.6 3 3 2 2.4 1.1 13.3 3 4 4 0.4 5.8 29.9 2 1.1 16.1 4 3 2.5 2.7 2.8 2.9 1 2 1 1 = 0 kg N hi 1 = 30 cm 2 = 67 kg N 11-1 2= 60 cm 3= 90 cm 1.3 0.5 4 29.2 6.8 24.1 0.5 3= 134 kg N hi 4 = 204 kg N 136 Appendix Table 11. Soil test results through winter months of clover-wheat rotation for 1995-96 growing season. NO3-N Mineralizable N Date of Soil depth NH4-N sampling cm mg kg' mg kg-1 mg kg' 10-27-95 2-16-96 0 Plots 0-30 cm 30-60 cm 60-90 cm 36-48 cm 6.6 0-30 cm 30-60 cm 60-90 cm 3.2 3.4 3.2 21.2 8.0 2.4 75.4 29.0 3.3 2.2 1.4 1.7 17.4 6.4 4.4 Before fertilizer was applied 2-16-96 Plot # 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 10/27/95 pH 5.1 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 4.5 4.7 4.5 3.6 3.4 3.7 4.4 3.9 3.8 3.6 3.6 5.6 3.8 3.9 3.7 Sample depth 0-12" P Mg ppm meg/100g 46.0 1.4 3.2 3.1 2.8 2.4 2.4 2.8 2.6 2.3 2.2 2.5 2.0 2.3 1.9 2.1 2.3 Na 17.2 16.8 18.8 17.1 16.0 19.3 16.5 16.0 15.6 14.0 16.7 16.9 18.1 13.7 15.8 OM So4-S meg/100g 0.08 5.77 10.4 137 Appendix Table 12. End of season soil test results of clover-wheat rotation for 1995-96 growing season Block Treatment NO,-N Mineralizable N Depth NarN 1 1 1 1 1 2 1 1 3 1 2 1 1 2 2 1 2 3 mg kg-' 2.8 2.9 mg kg-' mg kg' 3.2 0.9 0.4 11.0 3.1 3.5 2.1 10.7 0.4 4.0 0.0 2.3 2.7 1 3 1 1 3 2 1 3 3 1 4 1 1 4 2 1 4 3 1 5 1 3.1 1 5 2 3.8 1 5 3 2 1 1 1 2 1 3 2.7 2.3 2.5 3.6 2 2 2 2 2 2 2 3 1 2 2 2 2 2 2 2 2 3 2 3 3 4 4 1 3.1 2 2.6 4 3 5 5 5 2 3 3 3 1 1 1 2 3 3 3 2 2 2 3 2 3 3 3 1 3 3 2 3 3 3 3 4 4 4 5 3 3 3 3 2 2 1 5 1 2.9 2 3.1 3 1 3.1 3.9 2.3 2.8 3 1 1 2 2.8 2.9 2.3 4.0 2 3 1.1 0.2 3.6 1.1 0.2 2.8 0.6 0.0 3.0 0.9 0.2 2.9 0.8 0.3 4.8 1.5 0.0 10.4 4.4 0.0 13.8 5.9 0.0 17.2 5.9 0.0 12.9 7.0 0.0 11.3 4.4 0.0 16.4 6.9 0.0 11.5 6.2 0.0 11.9 6.1 0.0 3.3 13.7 5.5 1.1 2.4 2.8 3.5 2.4 1.0 3.3 1.4 3.2 3.5 12.9 7.1 0.5 0.3 3.3 1.2 0.4 2.6 0.8 3 1 0.5 2.6 0.6 0.1 2.0 0.5 0.1 3.0 0.9 0.2 3.9 6.4 0.0 0.0 12.1 8.0 0.0 18.4 6.3 0.0 12.7 8.5 0.0 0.4 Treatments 1 = 0 kg Nit' 2 = 56 kg N hi' 3 = 112 kg NW' 4 = 168 kg N11-15 = 224 kg N11-1 Depth 1 = 30 cm 2= 60 cm 3= 90 cm 3 5 138 Appendix Table 13. Soil test results through winter months of corn-wheat rotation for 1995-96 growing season Date of Soil depth NO3-N Mineralizable N NH4-N sampling cm mg kg-1 mg kg-1 mg kg-1 01/12/96 0-30 cm 30-60 cm 60-90 cm 36-48 cm 4.5 2.5 2.7 2.4 4.5 5.9 21.3 13.0 03-06-96 0 Plots 0-30 cm 30-60 cm 60-90 cm 12.9 12.9 14.5 4.5 8.8 7.2 45.6 16.8 24.1 Before fertilizer was applied 2-13-96 Plot # 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 5.1 4.1 2.2 5.8 7.0 5.0 8.7 1.8 1.8 1.8 8.1 9.3 2.9 3.0 1.7 1.9 1.7 1.8 4.8 3.4 8.1 4.7 3.4 5.7 7.0 25.0 24.9 28.6 26.5 27.0 34.3 20.7 28.0 24.4 22.7 34.4 27.4 25.0 27.4 24.1 2.1 2.3 2.0 3.6 2.3 01/12/96 pH 6.0 Na meq/100g % OM meq/100g Mg meq/100g 14.6 0.14 0.14 6.9 P ppm K Ca ppm 50.0 406 139 Appendix Table 14. End of season soil test results of corn-wheat rotation for 1995-96 growing season Block Treatment Depth 1 1 1 1 1 2 1 3 2 1 2 2 2 1 3 1 1 3 2 1 3 3 1 2 1 4 4 4 1 5 1 1 5 5 2 1 1 1 2 1 3 2 2 2 2 1 1 1 1 1 2 2 2 2 2 2 2 2 2 2 2 2 2 2 1 NO,-N mg kg-1 mg kg-1 mg kg-1 1.8 16.7 7.7 2.8 3.2 3.7 3.4 3 1 3.3 3.6 3.2 3.8 3 4.1 2.8 3 1 2.7 3.2 3.6 2.5 3 3 1 3 2 3 3 4 4 4 5 1 2.9 2.7 0.7 0.5 1.8 19.9 0.8 0.3 0.7 0.4 0.2 0.9 0.5 0.2 7.8 18 15.7 8.5 0.3 3 0.4 0.7 0.4 6.9 1 3.9 3.3 1 2 5 3 3 1 1 3 1 2 3.1 4.8 0.4 0.8 0.5 3 1 3 3 1 3 2 3.2 3 2 2 2 3 3 1 3 3 2 3 3 3 3 3 2.9 2.9 15.6 7.7 8.6 2 3 10.2 1.8 5 3.4 3.2 20.4 0.7 0.7 0.8 0.6 0.5 0.7 0.3 1.2 0.5 0.3 1.6 2 Mineralizable N NH4 N 13.9 18 18 7.7 19.6 11.4 0 14.4 10.1 0.1 0.6 0.1 0 20.8 7.3 1 20.6 0.3 12.5 0.2 1.6 23.3 12.2 0.5 3 3 0.1 20.4 1 2.2 3 5 4.2 3 5 10.9 3.3 0.9 2 3 0.4 3 5 Treatments 1 = 0 kg N 11-1 2 = 56 kg N hi 3= 112 kg N11-1 4 = 168 kg N11-1 5 ---- 224 kg N If' Depth 1 = 30 cm 2= 60 cm 3= 90 cm 3 3 4 4 4 1 2 4.5 4.6 140 Appendix Table 15. Soil test results through winter months of grass-wheat rotation for 1995-96 growing season Date of sampling 10-27-95 Soil depth cm NH4-N 0-30 cm 30-60 cm 60-90 cm 36-48 cm 4.5 2.5 2.7 mg kg-1 NO3-N mg kg-1 Mineralizable N mg kg' 11.7 5.5 2.6 2.5 76.1 22.5 2.7 2.9 3.2 3.0 35.2 50.5 38.4 38.4 29.0 34.3 31.4 43.6 28.0 33.9 37.2 37.3 25.6 34.7 28.7 Before fertilizer was applied 2-14-96 Plot # 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 0-30 cm 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 pH 6 3.5 3.9 4.5 3.0 4.9 4.7 5.0 5.0 3.4 4.2 3.3 3.7 2.9 3.9 3.2 1.9 2.8 2.8 2.5 2.3 2.4 2.9 2.3 2.3 2.4 2.5 Na ppm ppm Mg meq/100g meq/100g 61 172 1.3 0.07 % OM So4-S 5.58 7.1 141 Appendix Table 16. End of season soil test results of grass-wheat rotation for 1995-96 growing season. Block NO,-N Mineralizable N Treatment Depth NI-14-N mg kg' mg lie mg kg' 1 1 1 1 1 2 1 1 3 1 1 2 1 2 2 2 1 3 1 1 3 2 1 3 3 1 1 4 4 2 1 4 3 1 5 1 1 5.2 3.3 5.3 5.3 3 4.8 3.8 4.90 5.30 1.20 0.75 4.7 2 3.1 30.20 10.50 4.10 5.30 1 1 2 2 2 2 2 2 2 2 2 2 2 2 2 2 1 2 1 3 2 2 2 2 3 1 4.5 3.8 4.50 8.70 1.50 0.80 23.80 5.80 0.70 32.50 9.90 4.70 7.60 3 1 4.8 3 2 4 3 3 4 4 4 2 5.4 3.8 3 1 2 4.3 2.5 3 3.9 4.2 1 1 3 1 2 3 1 3 3 1 3 2 2 2 3 2 3 3 3 1 4.1 3 3 2 4 3 3 3 3 4 4 4 2 1 4.4 3.2 4.8 3.6 3 3 5 5 2 3 5 3 1 1.10 0.75 3.9 2.7 3 1 40.50 3.60 32.20 3.30 24.80 4.50 52.30 3.90 1.60 3 3 1.10 1 1 3 0.75 4.80 21.00 6.60 2 3 1.10 4.9 3.9 1 5 5 5 5 5 7.20 4.6 3.4 1.10 0.75 1.70 0.90 6.30 1.40 0.90 5.30 1.40 1.20 13.00 2.90 1.60 19.60 3.50 1.80 60.10 8.20 17.70 3.00 21.00 5.20 30.10 2.80 22.90 4.80 41.40 4.50 16.20 1.50 0.00 45.10 6.20 15.90 1.30 14.80 3.20 21.80 4.10 Treatments 1 = 0 kg N fil 2 = 56 kg N11-1 3 = 112 kg N 11-1 4 = 168 kg N hi 5 = 224 kg N Fr' Depth 1 = 30 cm 2= 60 cm 3= 90 cm 142 Appendix Table 17. Agronomic data of Hyslop Farm small plot research site for 1995-96 growing season. 6-in sample Block Treatment Rotation 1 1 1 2 1 1 3 1 1 4 1 1 1 1 2 1 3 1 2 2 2 2 4 1 1 2 2 2 2 2 2 2 3 2 4 2 2 2 1 2 3 4 1 1 1 1 2 2 1 3 1 2 3 1 3 3 1 4 3 1 1 3 2 3 Straw Kg/ha N% 2591 4198 2460 3772 4986 2895 5855 2394 5437 5453 7470 2435 8905 9610 9553 9602 10562 8856 8446 9414 11160 0.29 0.25 0.23 0.29 0.26 0.28 0.26 0.23 0.25 0.21 0.22 0.23 0.22 0.25 0.23 0.24 0.25 0.19 0.24 0.21 10381 7191 0.27 0.28 0.29 0.35 0.34 0.25 0.29 0.35 0.26 0.25 0.27 0.43 0.33 0.38 0.33 0.53 0.5 0.41 0.36 3 3 2 2 2 4 3 2 7528 1 4 1 2 4 1 3 4 4 4 4 4 4 4 1 5 1 2 5 1 3 5 1 4 5 1 1 5 2 5 2 2 3 5 5 2 2 10545 11882 5166 10365 10652 8971 11037 11431 11136 9471 10758 15252 11603 9766 16064 11373 4 1 2 3 4 Rotation : Treatment : 1 1 2 2 2 2 0.3 Grain Kg/ha N % 1845 3067 2075 2632 2870 2132 4535 2895 3756 3985 4822 3813 6527 6084 6880 6642 7175 6199 5929 6765 8725 7708 5314 5912 7273 9299 4100 8307 5396 7060 6732 9135 8208 7396 7970 8774 8102 9733 12439 8823 Yield Kg/ha Hole plot Protein Tw (g/qt) (%) 1.30 1.83 1.75 1.57 2231 2375 2464 2119 3984 3034 4790 2978 4427 3375 3741 3830 4666 5361 5876 5537 5872 5853 6004 5439 6560 6613 6723 7823 7000 7449 7703 6272 7114 6844 7762 7176 7639 7821 8255 8094 9043 8748 8038 873 866 871 865 868 853 872 872 856 850 836 841 855 859 861 858 850 845 857 844 867 862 866 857 858 853 853 855 862 870 864 872 865 865 847 868 870 858 872 1.49 8343 859 1.43 1.51 1.47 1.57 1.53 1.53 1.56 1.68 1.31 1.24 1.28 1.27 1.57 1.51 1.40 1.56 1.40 1.28 1.33 1.40 1.56 1.63 1.50 1.46 1.32 1.44 1.42 1.42 1.54 1.43 1.47 1.54 1.48 1.47 1.54 1 = Oat-wheat 2 = Clover-wheat 1 =0 kgN11-1 4= 150 kg N 2 = 50 kgNlil 3 = 100 kg1\1111 5 = 200 kg N 8.43 8.58 8.61 8.13 8.45 8.64 8.46 8.86 8.12 7.36 7.76 7.17 8.30 8.51 7.94 8.36 7.98 7.71 7.90 7.20 8.56 8.49 8.12 8.39 8.03 7.91 7.84 7.62 8.17 8.23 8.10 8.34 8.37 8.25 8.47 8.41 8.91 9.07 8.44 8.25 143 Appendix Table 18. Agronomic data of Hyslop Farm small plot research site for 1996-97 growing season. 6-m sample Block Treatment Rotation 1 1 1 2 1 1 1 3 1 4 1 1 1 1 2 1 3 1 4 1 2 2 2 2 1 2 1 2 2 1 3 1 1 2 2 2 2 2 3 4 2 2 2 2 2 2 4 1 1 3 1 2 3 1 3 3 1 4 3 1 1 3 2 2 3 2 3 3 2 4 3 2 1 4 4 4 4 1 4 4 2 2 2 2 2 3 4 1 2 1 1 1 1 4 4 5 2 5 1 3 5 5 1 4 1 5 2 2 5 3 5 2 2 4 5 2 3 4 Rotation : Treatment : 1 1 1 = Oat-wheat Straw Kg/ha 1673 1624 1591 1886 5807 6324 5643 4995 6389 5495 4446 7603 9129 14985 11713 7644 12680 8604 10187 9662 12352 11376 14321 11901 9613 10696 11393 9408 13739 13607 12156 12680 11138 11762 8661 12680 13148 13181 12393 N % Kg/ha 0.20 0.35 0.26 0.23 0.17 0.15 0.17 0.18 0.13 0.15 0.16 0.12 0.15 0.16 0.15 0.22 0.14 0.17 0.19 0.17 0.23 0.29 0.25 0.22 0.23 0.32 0.13 0.14 0.30 0.26 0.35 0.32 0.36 0.33 0.36 0.31 0.46 0.44 0.39 0.52 10843 2 = Clover-wheat 1 = 0 kg N hi' 2 = 50 kg N 11-' 4= 150 kg N hi 5 = 200 kg N h1 3 Hole plot Tw Protein (%) Kg/ha (g/qt) Yield Grain N% 1288 1043 1024 1190 9621 9137 7439 8727 8612 7833 7308 10105 10220 10622 8801 1.14 1.34 1.25 1.27 1.22 1.26 1.31 1.40 1.09 1.15 1.10 1.20 1.19 1.25 1.30 1.50 1.16 1.17 1.26 1.28 1.38 1.33 1.37 1.48 1.31 1.63 1.29 1.46 1.55 1.48 1.51 1.59 1.46 1.80 1.50 1.72 1.77 1.73 1.71 2839 3756 3218 3815 3022 2833 2594 3025 4967 5517 5889 4851 4909 4464 4596 5398 6312 6849 7061 6179 5194 6188 6261 6675 7712 7997 7984 7780 6961 7209 7305 8070 7140 7843 7689 840 836 834 837 831 842 841 836 827 820 825 824 838 837 841 846 839 837 831 833 849 852 856 860 843 847 839 846 865 859 858 869 850 871 849 862 865 870 864 6709 1.87 6998 857 1083 1058 1468 1476 3781 4101 4101 2928 4158 3617 3150 5331 5307 6972 6767 4388 8514 6357 6660 6316 7882 8317 9719 7390 8432 8801 7767 8374 = 100 kg N 11-' 7.5 7.9 7.7 7.8 7.9 8.2 8.3 8.3 6.7 7.2 6.8 7.2 7.7 7.9 7.9 8.4 7.2 7.2 7.1 7.1 8.0 8.2 8.2 8.5 8.1 8.1 7.4 7.8 8.6 8.4 8.9 9.2 8.1 9.3 8.3 8.7 9.5 9.4 9.8 10.1 144 Apendix Table 19 Biomass and N uptake of five spring wheat varieties (1997) Date : 1 = April 25 2 = May 13 3 = May 31 4 = June 22 5 = July 6 = 11 August 1 Variety : 1 = Alpowa 2 = Pennawawa 3 = Treasure 4 = WB963R 5 = White bird 1[GDD 218 218 218 218 218 218 218 218 218 218 218 218 218 218 218 218 218 218 218 218 467 467 467 467 467 467 467 467 467 467 467 467 467 467 467 467 467 467 "G Stage 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 Block 1 2 Date Variety 1 1 1 1 1 1 4 1 1 1 1 2 1 2 2 3 1 2 4 1 2 1 1 3 2 1 3 3 1 3 4 1 3 1 1 2 1 3 1 4 1 4 4 4 4 1 1 5 2 1 5 3 1 4 1 5 5 5 1 2 1 5 2 3 5 4 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 1 5 1 5 2 5 3 5 4 5 1 5 2 5 3 5 4 5 1 5 2 5 3 5 4 5 1 5 2 N uptake kg ha' kg ha-1 63 3 5 Biomass 49 46 52 89 60 66 52 60 75 69 69 63 77 83 1 98 46 69 52 66 809 525 565 574 2 712 2 2 763 1 3 732 548 910 772 769 709 4 1065 4 832 887 852 784 594 2 3 3 3 4 4 5 5 3.3 2.1 2.3 2.2 4.8 2.9 2.7 2.5 3.3 3.4 3.3 2.9 3.7 3.4 4.4 4.0 2.7 3.2 2.3 2.4 31.7 20.3 24.2 22.4 30.4 36.7 28.5 22.9 33.4 28.7 27.5 23.4 45.9 30.8 34.4 27.1 29.8 26.5 145 Appendix Table 19 (continued) 1GDD IIG Stage Block Date Variety Biomass N uptake kg ha' kg ha' 5 827 29.5 4 2 2 5 1 3 1 6 2 3 1 6 6 3 3 1 4 3 1 6 1 3 20.5 76.5 85.2 70.5 71.6 74.0 6 2 3 6 6 3 3 4 3 2 2 2 2 6 1 3 3 6 6 6 6 2 3 3 3 3 3 4 3 3 1 3 4 6 2 3 6 6 6 3 3 4 3 4 4 4 1 3 5 763 763 763 6 2 3 5 6 3 3 5 6 4 1125 1125 1125 1125 1125 1125 1125 1125 1125 1125 1125 1125 1125 1125 1125 1125 1125 1125 1125 1125 1433 10 10 10 10 10 10 10 10 10 583 2959 3499 2904 3160 3257 4156 2982 3900 3252 3381 3788 2767 4951 5266 4380 4018 3941 3369 2787 2821 9988 7376 10102 9041 7893 9815 8840 7806 6802 9098 7893 7950 467 5 3 467 763 763 763 763 763 763 763 763 763 763 763 763 763 763 763 5 6 763 763 3 5 4 4 1 1 2 4 4 4 4 3 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 1 5 1 2 3 4 1 1 10 10 10 10 2 10 10 10 2 10 10 10 10 10.5 3 4 1 3 4 1 2 3 1 1 2 2 2 2 3 3 3 3 4 4 4 4 5 5 5 5 1 10361 10189 9959 8409 7376 9902 8983 8467 12599 112.7 88.8 97.7 79.6 78.2 105.7 51.7 96.5 128.7 97.6 96.4 79.7 78.8 62.5 69.1 119.9 74.4 140.2 123.8 113.2 91.7 190.1 213.0 123.7 171.9 128.0 161.5 125.6 115.9 160.7 135.0 82.1 127.5 131.7 132.7 142.0 146 Appendix Table 19 (continued) 1GDD "G Stage Block 1433 1433 1433 1433 1433 1433 1433 1433 1433 1433 1433 1433 1433 1433 1433 1433 1433 1433 1433 1784 1784 1784 1784 1784 1784 1784 1784 1784 1784 1784 1784 1784 1784 1784 10.5 10.5 10.5 10.5 10.5 10.5 10.5 10.5 10.5 10.5 10.5 10.5 10.5 10.5 10.5 10.5 10.5 10.5 10.5 1784 1784 1784 1784 1784 Date Variety 2 5 1 3 5 1 4 5 1 1 5 2 5 3 5 4 5 2 2 2 2 1 5 3 2 5 3 3 5 3 4 5 3 1 5 2 5 3 5 4 5 4 4 4 4 1 5 5 2 5 5 3 5 5 4 5 5 11 1 2 11 3 11 4 11 1 11 2 6 6 6 6 6 6 1 11 11 3 6 11 4 11 1 6 6 11 2 11 11 1 1 1 2 2 2 2 3 3 3 6 6 4 6 3 11 1 6 11 2 6 11 3 11 4 6 6 6 6 6 6 4 4 4 11 1 11 2 11 3 11 4 1 Growing degree days " Feekes growth stage 3 4 5 5 5 5 Biomass N uptake kg ha' kg ha-1 12800 10533 9586 15125 16474 11394 12198 12111 16158 11911 14063 11337 16158 10476 11738 11509 13001 11824 11423 11214 10590 12690 10562 13690 13000 14041 13805 13633 14723 14051 14264 11853 12341 12653 12886 14379 12838 10339 10935 109.1 110.4 92.1 167.0 152.3 140.0 160.3 130.7 183.9 136.7 153.4 132.5 145.6 130.5 132.3 126.4 134.9 118.9 134.5 117.4 108.0 130.3 111.6 154.6 143.0 163.8 159.0 150.0 148.7 151.7 156.9 118.5 146.4 127.8 141.8 138.5 125.8 122.0 126.9 Appendix Table 20 Data from on-farm-mini plots (1995-96) Soil Minralizable ¶ Total Soil N at Growers Name Feeks 5 N at Feeks 5 Plant N uptake at Feeks 5 ¶ Total Soil N at harvest Plant N uptake at maturity kg ha -1 mg kg-1 Sam Sweeney 26.7 27.4 26.8 20.0 96 1 2 Hamlin Farms 28.0 25.8 36.7 33.9 110 Dalke Farms Wilco farm 22.0 25.8 13.6 25.8 87 3 Steve Vanger 15.1 47.0 20.2 16.8 59 4 Tom Cranford (Susan Aldrich) 25.2 26.6 22.9 16.4 82 5 Pat Peters 17.2 29.6 28.4 9.0 50 6 Dave Jossi 23.8 27.0 29.9 29.4 75 7 Forest Hills farms 23.2 31.9 21.4 20.8 86 8 Handrics Farm (Paul Camuso) 17.0 37.2 11.3 28.9 50 9 Mark Shmdlin Hard drough 17.2 42.1 30.1 15.1 42 10 11NO3-N and NH4-N