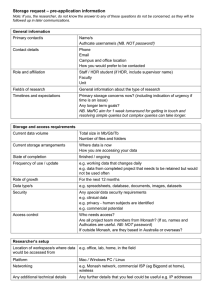
Safety Information for Staff and Students
advertisement

School of Biological Sciences Safety Information for Staff and Students January 2013 Table of Contents 1 Introduction ........................................................................................ 1 2 Emergency Contact Officers ................................................................ 2 3 Emergency Procedures ....................................................................... 5 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 3.1 3.2 3.3 3.4 School Safety Staff ....................................................................................... 2 School First Aiders ....................................................................................... 2 Emergency Compressed Air Breathing Apparatus ........................................... 3 Advanced Resuscitation ............................................................................... 4 Defibrillation................................................................................................. 4 OHS Contacts .............................................................................................. 4 Radiation Contacts ....................................................................................... 4 Field trip Safety Contacts .............................................................................. 4 If you are first on the scene of an emergency .................................................. 5 Evacuation .................................................................................................. 6 3.2.1 Building Information ...................................................................... 6 3.2.2 Weekend or After Hours Evacuation ............................................... 7 First Aid Procedures ..................................................................................... 7 Safety Aids .................................................................................................. 7 3.4.1 Fire Extinguishers ......................................................................... 7 3.4.2 First Aid Kits ................................................................................. 7 3.4.3 Safety Showers / Eye wash ........................................................... 8 3.4.4 Wheel Chair ................................................................................. 8 4 Security............................................................................................... 9 5 Other Safety-related Information ........................................................ 11 4.1 4.2 4.3 4.4 4.5 5.1 5.2 5.3 5.4 5.5 Contact phone numbers................................................................................ 9 Security and Traffic Office ............................................................................. 9 Theft ........................................................................................................... 9 Security Escort Service ................................................................................. 9 Campus Buses .......................................................................................... 10 4.5.1 Security Buses ........................................................................... 10 4.5.2 Car Park Shuttle Service ............................................................. 10 4.5.3 Inter-Campus bus ....................................................................... 10 Health Service ............................................................................................11 Pre-Existing Medical Conditions ...................................................................11 Insurance Cover For Students and Staff ........................................................11 Volunteer Workers.......................................................................................11 Children in the Workplace ............................................................................11 6 OHSE Training .................................................................................. 13 6.1 6.2 6.3 6.4 6.5 Induction and Essential Training .................................................................. 13 Specific Training......................................................................................... 13 Laboratory Inductions .................................................................................14 Training Aggreements................................................................................. 15 6.4.1 Training for Equipment ................................................................ 15 6.4.2 Training for Hazardous Substances .............................................. 16 Risk Assessments ...................................................................................... 16 7 Inspections and Audits ...................................................................... 17 8 Electrical Testing............................................................................... 19 7.1 7.2 8.1 8.2 In-house OHS Inspections...........................................................................17 External Audits ...........................................................................................17 7.2.1 OHS External Audits....................................................................17 7.2.2 Worksafe inspections ..................................................................17 7.2.3 OGTR Inspections....................................................................... 18 7.2.4 Quarantine Inspections ................................................................ 18 7.2.5 Drugs and Poisons Audit ............................................................. 18 Residual Currency Devices (RCD‟s) ............................................................. 19 Testing, Tagging and Repairing Electrical Equipment .................................... 19 9 Testing Emergency Eye wash and Safety Showers ............................. 21 10 Hazards and Risk Areas within the School ......................................... 22 11 Hazard & Incident Reporting & Investigation ......................................24 12 Risk Management .............................................................................. 25 13 Immunisation ................................................................................... 27 14 Safe Laboratory Practices.................................................................. 28 9.1 9.2 10.1 12.1 12.2 14.1 14.2 14.3 Testing Emergency Eye Wash ..................................................................... 21 Testing Safety Showers .............................................................................. 21 Health and Safety Issue Resolution..............................................................23 Responsibilities ..........................................................................................25 The Risk Assessment Process ....................................................................25 12.2.1 Helpful Hints .............................................................................. 26 What each Laboratory needs to do and have ................................................ 28 Personal Protective Equipment (PPE) .......................................................... 29 14.2.1 Footwear .................................................................................... 29 14.2.2 Protective Clothing / Lab Coats .................................................... 29 14.2.3 Eye Protection ............................................................................ 30 14.2.4 Hearing Protection ...................................................................... 30 14.2.5 Hand Protection .......................................................................... 30 14.2.6 Respirators ................................................................................. 30 Ergonomics in your Workspace 31 14.4 15 Chemical Safety ............................................................................... 34 15.1 15.2 15.3 15.4 15.5 15.6 16 14.3.1 Computer/Office Work Stations .................................................... 31 14.3.2 Laboratory.................................................................................. 31 14.3.3 Pipetting..................................................................................... 31 14.3.4 Microscope................................................................................. 32 14.3.5 Laboratory Hoods ....................................................................... 32 14.3.6 Other Laboratory Tasks ............................................................... 32 Unattended Experiments............................................................................. 33 Definitions and Legislative Requirements .....................................................34 15.1.1 Dangerous Goods.......................................................................34 15.1.2 Hazardous Substances ...............................................................34 15.1.3 Controlled Substances (Drugs and Poisons) ................................. 35 15.1.4 Purchase and Storage of Scheduled Poisons ................................. 35 15.1.5 Working with Scheduled Carcinogens ........................................... 35 Maintenance and Requirement of Chemical lists and Material Safety Data Sheets ....................................................................................37 15.2.1 School Responsibilities................................................................37 15.2.2 What does EVERYONE need to do? ............................................ 38 Storage Limits for Dangerous Goods in Laboratories ..................................... 38 15.3.1 Special Items.............................................................................. 38 15.3.2 Storage of Dangerous Goods in Refrigerators/Coldrooms.............................................................. 39 15.3.3 Gas Cylinders in the Laboratory ................................................... 39 Chemical Waste Disposal Procedures.......................................................... 41 15.4.1 User Responsibility ..................................................................... 41 15.4.2 Disposal of Chemical Waste ........................................................ 41 Spill Management Procedures .....................................................................42 15.5.1 Spill Kits .....................................................................................43 15.5.2 Mercury Spills.............................................................................43 15.5.3 Chemical Spill Response Procedure .............................................45 Weighing Toxic/Hazardous Substances ...................................................... 46 Biosafety .......................................................................................... 47 16.1 16.2 16.3 16.4 Operating Procedures................................................................................ 47 16.1.1 PC1 and PC2 Laboratories ......................................................... 47 16.1.2 Bacteriological Work ................................................................... 48 16.1.3 Class II – Biological Safety Cabinets............................................. 48 Biological Hazards and Spills ...................................................................... 49 16.2.1 Spills Outside a Biological Safety Cabinet ..................................... 49 16.2.2 Cleaning Up a Biological Spill ...................................................... 49 PC2 and Quarantine Glasshouses ............................................................... 50 16.3.1 Operating Procedures ................................................................. 51 16.3.2 Containment Precautions ............................................................ 51 Handling and Disposal of Products of Biological Origin .................................. 52 16.4.1 Compliance with AQIS Importation Guidelines............................... 52 16.5 17 16.4.2 Disposal of Quarantine Waste ......................................................52 16.4.3 Disposal of Biological Waste ........................................................52 International Air Transport Association (IATA) Regulations ............................ 54 Working with Radiation ..................................................................... 55 17.1 17.2 17.3 17.4 17.5 Useful contacts...........................................................................................55 Purchase of Radioisotopes ..........................................................................55 Storage and Use of Radioisotopes ...............................................................55 17.3.1 Radioisotope Laboratories ...........................................................55 17.3.2 Authorisation ..............................................................................55 17.3.3 Radiation Safety Procedures ....................................................... 56 17.3.4 Personal Radiation Monitors ....................................................... 57 17.3.5 Working outside normal working hours ......................................... 57 Accidents and Spills................................................................................... 57 Disposal of Radioactive Materials ................................................................58 18 Liquid Nitrogen ................................................................................. 59 19 Field Trip Safety ................................................................................ 60 18.1 18.2 19.1 19.2 19.3 20 Safety........................................................................................................ 59 Re-filling Dewars............................................. …………………………………………59 Communications......................................................................................... 60 19.1.1 Essential Planning....................................................................... 60 19.1.2 Communications ........................................................................ 61 Parking of Private Cars whilst on Field Trips ................................................ 61 Scuba Diving & Boating Safety ................................................................... 61 Equipment Safety, Training and Maintenance....................................62 20.1 20.2 20.3 20.4 20.5 20.6 20.7 20.8 20.9 20.10 20.11 20.12 Isolation of Unsafe Machinery or Equipment ............................................... 62 School Facilities ........................................................................................ 63 Autoclaves ................................................................................................ 63 20.3.1 Training..................................................................................... 63 20.3.2 Hazards, Faults and Maintenance ............................................... 65 Centrifuges ............................................................................................... 66 Fume Hood and Laminar Flow .................................................................... 66 20.5.1 Fume Hood Training................................................................... 66 20.5.2 Laminar Flow Training .................................................................67 Incubators and Ovens .................................................................................67 Constant Temperature (CT) Cabinets and Rooms .........................................68 Fridges and Freezers ..................................................................................68 Balances – Calibration and Cleaning ............................................................68 Pipettes .....................................................................................................69 20.10.1 Ergonomic Use ...........................................................................69 20.10.2 Calibration and Cleaning of Manual Plunger-Operated Pipettes ......................................................................................69 Specialised Laboratory Equipment ...............................................................70 Equipment Responsibilities and Training....................................................70 20.12.2 21 Equipment Training ................................................................................... 70 Policies, Procedures & Guidelines………………………………………….72 21.1 Emergency Response Procedures –Business Hours.....................................72 21.2 Emergency Response Procedures – After Hours.......................................... 73 21.3 Fire Extinguishers ......................................................................................74 21.4 First Aid Treatment .................................................................................... 75 21.5 Screen-Based Equipment Guidelines .......................................................... 76 21.5.1 Screen-Based Equipment Exercises .........................................................77 21.6 Insurance .................................................................................................. 78 21.6.1 Student Personal Accident Insurance Policy.................................. ……………….78 21.7 Use of headphones, earphones, ear buds in the Workplace ........................... 79 21.8 Other OHSE Policies and Documents .......................................................... 80 1 1 Introduction Under the Occupational Health & Safety Act (1985), the University is required to provide a healthy and safe working environment for all employees and students and to promote safe working practices. In fulfilling this obligation, the School of Biological Sciences adheres to the policies of Occupational Health, Safety and Environment (OHSE) at Monash University. The School also requires that staff and students conduct themselves in a responsible manner when working in all areas of the school, and comply with any instructions given to them by the Safety Officer or other responsible staff. The School has an OHSE Committee that meets four times per year. The Head of School is the Chair of this Committee. The information in this manual provides details on various safety issues that will affect you during your time with the School. Please keep this manual where it is readily accessible and refer to it as required. It is only a general reference manual, and should not be regarded as a substitute for training and for consulting with appropriate personnel. Further information may be obtained from the following locations: School of Biological Sciences (V drive): V:\OHSE Faculty of Science: http://www.sci.monash.edu.au Occupational Health, Safety and Environment branch http://www.monash.edu.au/ohs/ “Ask Monash” is an on-line service which allows you to find out the answers to your health and safety questions (and other questions) quickly and conveniently by searching a database of frequently asked questions. If a suitable answer cannot be found, you can submit the inquiry to OHSE for resolution. http://www.adm.monash.edu.au/askmonash/about.html 1 2 Emergency Contact Officers 2.1 School Safety Staff Position Name Extension Room Safety Officer Leesa Hughes 20384 18-104a Health and Safety Representative John Arvanitakis 53877 18-104a 20384 18-104a 17-235 First Aid Co-coordinator Leesa Hughes Radiation Safety Officer Prof John Beardall 55601 Biosafety Officer Dr Richard Burke 59531 Environmental Officer Leesa Hughes 20384 53-109 18-104a 2.2 School First Aiders The following members of staff have received formal training in First Aid, and can be contacted at any time when assistance is required. Please note a number of our first aiders are also trained in Mental Health First Aid please contact one of the Safety Officers for more information. Building Number Building 17 Building 18 Building 22 Building 53 * Mental Health First Aid Trained Staff Member Rohan Clarke Tim Cavagnaro Cecilia Blomstedt Nga Dang-Lien * Christa Meek* Caitlin Johnston * David Chapple Richard Reina Bruce Weir Jodi Neary Sherrie Caarels Emily Skoda Ricardo San Martin John Arvanitakis Leesa Hughes * Gerry Rayner Yardenah Brickman Chris Wilson * Inaki Iturbe-Ormaetxe Jyotika DeBruyne Nichola Kenny Extension 51968 55793 54481 51511 51511 51511 53015 55600 55660 50512 51637 24630 55637 53877 20384 55629 55671 55624 20177 20101 50049 Room Number G16 233a 317 337 337 337 G19 G20 112 114 114 114 117 104a 104a 118 G06 G06 106 116 136 After hours contact for first aid assistance: Security Ext 333 , Security from Mobile phone 99053333 2 2.3 Emergency Compressed Air Breathing Apparatus Two units are located on the second floor of building 17, opposite the lift entrance. Staff currently trained in the use of Breathing Apparatus are: Building Number Building 18 Staff Member John Arvanitakis Leesa Hughes Sherrie Caarels Emily Skoda Extension Number 53877 20384 51637 24630 Room Number 104a 104a 114 114 3 2.4 Advanced Resuscitation This must be available when anyone is doing any work that may generate cyanide gases or any diving. It can be booked through the Netscape calendar. Staff currently trained in the use of SOS apparatus are: Building Number Building 17 Building 18 Staff Member Ee Ling Ng Bruce Weir Jodi Neary Richard Reina Ricardo San Martin Sherrie Caarels Jyotika Taneja De Bruyne Nichola Kenny Building 53 Extension Number 55680 55660 50512 55600 55637 51637 20101 20101 Room Number 137 112 114 G20 117 114 115 115 2.5 Defibrillation The nearest defibrillator is located in the Faculty of Science Student Services Reception Building 19 room G26A. Security also have a unit and should be contacted if a unit is needed out of normal working hours. 2.6 OHS Contacts Biological Sciences OHSE consultant Debra Bartolo ext. 50222 or Debra.Bartolo@monash.edu General OHS branch ext. 51016 or ohsehelpline@monash.edu 2.7 Radiation Contacts Prof John Beardall Radiation Safety Officer (RSO) ext. 55611 John Arvanitakis Buildings and Infrastructure Officer ext. 53877 Margaret Rendell OHS&E Radiation Protection Officer ext. 51016 2.8 Field trip Safety Contacts Mr. Ric San Martin School Boating and Diving Safety Officer 18-117 ext. 55637 Mr. Bruce Weir Deputy School Boating and Diving Safety Officer 18-114 ext. 55660 4 3 Emergency Procedures 3.1 If you are first on the scene of an emergency If you are first on the scene of an emergency: For fire, activate a break glass alarm Lift a Red ‘E’ phone and advise Security For medical emergencies during normal hours, contact a first aider or lift a Red ‘E’ phone and advise Security If hazardous substances, radiation or biological materials are involved during normal working hours, contact the Safety Officer If a serious accident occurs involving; fire, toxic gas or chemicals, it is important to activate the nearest fire alarm immediately. This: Shuts down the plenum ventilation, thus preventing the dispersion of fumes throughout the building Activates the fire alarm, notifying Security and the Fire Brigade Sounds the building alarm, prompting the wardens to evacuate the building After activating the fire alarm, immediately turn off any electrical equipment you are using and then, if time permits, notify the School Manager (ext. 55610) of the nature of emergency and then follow evacuation procedures. The Biology and Zoology Building, S7/S8 Lecture Theatres, 1st Year Biology and the Zoology Annexe are on three separate emergency evacuation alarm systems. This means that one building may have to evacuate when the others do not. Please find detailed below the different sets of emergency procedures for these buildings. Please be sure you know what to do, depending which building you are in. 5 3.2 Evacuation 3.2.1 Building Information Building Warden Deputy Warden (s) Assembly area Biology (17) Mary Pantzikis TBA The grassed area to the north of building 17, between the Workshop and Microbiology. Zoology (18) Jodie Weller TBA Lawn area adjacent to First Year Chemistry building (not in the undercroft!) First Year Biology (22) and Lecture theatres S7 & S8 (21) Chris Wilson TBA Lawn area adjacent to First Year Chemistry building (not in the undercroft!) NB: The Zoology Annexe is on the evacuation system controlled by Central Sciences (OHS Zone 13). The Building Warden from Building 18 can communicate with the Floor Wardens in that area and give instructions. In addition, wardens are assigned to each floor of each building, to ensure that evacuations proceed smoothly, correct procedures are followed and all personnel vacate the building. ON THE ALERT TONE (regular beeping) prepare to leave: LISTEN FOR ANNOUNCEMENTS OVER THE PUBLIC ADDRESS SYSTEM Turn off all electrical equipment Store hazardous materials safely Close doors and windows DO NOT EVACUATE UNTIL YOU HEAR THE EVACUATE TONE ON THE EVACUATE TONE (a whooping noise) leave the building by the nearest available emergency exit: Follow instructions from floor wardens or emergency personnel Do not lock your lab or office door as you leave - in the event of a genuine emergency this will serious hamper rescue efforts. DO NOT USE THE LIFT Go to the nominated assembly area away from buildings, and DO NOT leave that area until you are advised by the wardens that you may do so, even if the alarm tone stops sounding. DO NOT DELAY OR PUT YOURSELF AT RISK 6 3.2.2 Weekend or After Hours Evacuation If the emergency evacuation system is activated outside normal working hours, an ALERT tone is sounded. If no intervention occurs within two minutes the evacuation signal is automatically activated and Security is alerted. If this should occur, you should: Secure your work area turning off all electrical equipment, close but do not lock doors and windows If using a fume hood, ensure that it remains ON Leave the building by the nearest available emergency exit, using stairs NOT lifts Assemble in designated areas 3.3 First Aid Procedures For a quick-reference guide to the correct procedures to be followed if an accident or emergency occurs; please refer to the flow charts in sections 19.1 and 19.2 of this Manual. 3.4 Safety Aids 3.4.1 Fire Extinguishers These can be found in the corridors and laboratories. Please be aware what extinguishers are in your area and under what circumstances each particular type of extinguisher should be used (see section 19.3). Courses on the correct use of these fire extinguishers are available during the year, and all members of School will be advised of the dates. All staff and students are required to attend one of these courses when they first join the School and then at least once every five years thereafter. 3.4.2 First Aid Kits These are placed at strategic locations throughout the School and in all School vehicles. Kits are for emergency use only. Basic kits are located in each laboratory or in close proximity to laboratories, and a more comprehensive kit is located in a signposted central location on alternate floors. Please advise the technician in charge of your area or the First Aid Coordinator if you use any item from these boxes so that replacements can be organised. A list of first aid trained staff, together with room and phone numbers, is contained in this manual and will also be posted on each emergency evacuation floor plan. In addition the School has: Two extended “remote area” field trip first aid boxes. These are more comprehensively fitted out and are intended for staff and students on longer field trips in areas where the nearest medical assistance is some distance away A number of small “bum bag” first aid kits for use on undergraduate field trips 7 The remote area kits can be booked via the online calendar. Both the remote area kits and the bum bags can be collected from the First Aid Co-coordinator. Upon return from any trip, please report to the First Aid Co-coordinator any items which will need replacement. 3.4.3 Safety Showers / Eye wash These are located in the various locations around the school. Please know the location of the closest safety shower and eye wash facility. Water in copious amounts is the best antidote for any chemical splashes on you or in your eye. In the case of a chemical splash to the eye, irrigate immediately with running water (including under the lids) for at least 20 minutes and seek medical attention. All labs should make sure that this can readily be done with taps and hoses available. 3.4.4 Wheel Chair A wheel chair is located on the 1st floor, near 17-143 and the vending machines. Push glass to access the key. Please ensure the chair is returned after use. 8 4 Security 4.1 Contact phone numbers Emergency 333 or 53059 4.2 Security and Traffic Office The office is located in Building 61 and is operational 24 hours per day, seven days a week. They can be contacted by: Using the Red E phones Dialing 333 from any internal phone Dialing 9905-3059 from any external or mobile phone 4.3 Theft Unfortunately, theft is a common occurrence throughout the University. To help prevent it happening to you: Keep valuables (bags, wallets, laptop computers, mobile phones, etc.) under lock and key at all times. Always lock your office or laboratory when leaving, even during normal working hours and even if you will only be away for a short time. There have been instances in this School of laptops being stolen from desks during working hours when their owner went to a lab across the corridor for a few minutes! Report any suspicious occurrences to the School Manager, even if you are not certain whether anything was stolen. 4.4 Security Escort Service Clayton security patrols are available each night to escort you to your car, bus loop, Halls of Residence or other locations on Campus. The service is free. To arrange it, contact Security on ext. 53059. 9 4.5 Campus Buses 4.5.1 Security Buses A free security bus service runs on the Clayton campus in the evenings, Monday to Friday, all year round. Bus stops are clearly marked on a Clayton Campus Map. The first service leaves the Main Library at 5.30pm and buses run every 30 minutes until midnight. It runs a short distance into the residential areas near the western and southern side of the University. For more information on all of these services, see the webpage at: http://www.fsd.monash.edu.au/security/your-campus Or contact the Clayton Campus Services Manager on 9905-4082 4.5.2 Car Park Shuttle Service For those people who find they have to park in the “free park” on Blackburn Rd, there is a shuttle bus service which runs from 7.45am to 6.45pm. The pick-up point is at the Blackburn Rd entrance where there is also a phone in case of emergency. 4.5.3 Inter-Campus bus A shuttle bus runs approximately every thirty minutes between the Clayton and Caulfield campuses. The departure points are: Clayton: Wellington Road bus loop Caulfield: bus shelter in Queens Avenue http://fsd.monash.edu.au/travel-parking/travel/inter-campus-shuttle-bus 10 5 Other Safety-related Information 5.1 Health Service This is located in the Campus Centre (Building 10), at the west end of the central concourse, and is open during normal working hours. 5.2 Pre-Existing Medical Conditions Staff and students are requested to advise the school of any pre-existing condition which may be relevant to your evacuation in an emergency, or to your treatment in the event of a medical emergency. In accordance with current Privacy legislation, the provision of this information by you is voluntary. However, it is in your own best interests to supply any relevant information so that the School can ensure that appropriate action is taken. 5.3 Insurance Cover for Students and Staff See the Biological Science School Guide and section 21.6.1 of this manual. 5.4 Volunteer Workers All volunteer workers MUST register with the School Manager, to ensure that they are aware of all relevant OH&S requirements and are covered by appropriate insurance policies. Provided this is done, voluntary workers are covered under the University’s Personal Accident Insurance Policy. The coverage differs for those who earn an income and those that do not. A copy of the policy can be obtained from the School Manager. In addition, registered volunteers are covered by the University’s public and general indemnity insurance policies. 5.5 Children in the Workplace The School recognises that despite the range of childcare options available at the University, in order to meet their work and/or study commitments staff members and students may occasionally, in unforeseen situations, temporarily need to bring children to their workplace. However, when children are introduced to environments which are not designed to cater for them, issues of safety, supervision, productivity and legal liability arise. 11 All members of the School are strongly advised whenever possible to avoid bringing children into any of the School’s buildings. When it is essential to do so, please refer to the relevant University information at: http://www.monash.edu.au/ohs/topics/hazard-alerts/children-on-campus.html 12 6 OHSE Training The Occupational Health Safety & Environment branch conducts a number of training courses throughout the year. The Safety Officer is responsible for maintaining a database of the courses attended by staff and students and will liaise with individuals regarding the training as required. 6.1 Induction and Essential Training The School is required to provide a formal induction to all new members of the School (including staff, students and visitors) when they first join the School. Induction Sessions are organised in February-March and July of each year for commencing research students. All new staff members, including casual and sessional staff, should receive a folder of induction information, and should also be personally introduced to their work area and key staff by their supervisor. Monash Faculty of Science provides an induction checklist at: http://sci.monash.edu/safety/docs/facultycheck.pdf For information on Laboratory Inductions, see section 6.3 below. If you did not receive a formal induction, or if you feel it was inadequate in any way, please discuss it with your supervisor, the Safety Officer, or the Head of School. All members of School are strongly encouraged (and in some cases may be required) to undertake the following courses conducted by OHSE: Risk Management Chemical Safety 6.2 Specific Training The school has a very diverse range of research and teaching activities. Depending on the type of work you are involved in, it may be necessary for you to undertake a number of different courses in order to comply with University policy. The School is required to ensure that a suitable number of staff are trained as First Aiders (either level 1 or level 2), and in other OHS roles, to cope with any situations that may arise during normal teaching and research activities. The School will arrange the necessary training and updates as required, and the dates will be publicised through the School. If you are interested in being trained in any of these roles, please discuss it with your supervisor or the Safety officer. The available courses include: Emergency Warden Training and Evacuation Exercises First Aid Level 1 - Basic Life Support First Aid Level 2 - Emergency First Aid Breathing Apparatus Radiation - Unsealed sources 13 Radiation - X-ray, Irradiating Apparatus & Sealed sources Biosafety Oxygen Resuscitation Safety Inspections and Audits Training of First Aiders A list of the School’s trained OHS staff is in section 2 of this Manual. For further information, see: http://www.monash.edu.au/ohs/management-system/training-at-monash.pdf 6.3 Laboratory Inductions A Laboratory Induction should be completed BEFORE the commencement of laboratory work. The supervisor or designated qualified person should run through the “Supervisor‟ section of the Monash OHSE induction checklist: http://www.monash.edu.au/ohs/training/safety-induction-program.html If the Supervisor needs further information to assist in explanation of any points on the checklist, they should consult the appropriate section of this manual or ask the Safety Officer. Additional points to cover during the induction are: Is the laboratory a PC2 (Physical Containment level 2) facility? If so, read the PC2 sign on the door and familiarise yourself with the rules of a PC2 laboratory. PC2 facilities are bound by OGTR (Office of Gene Technology Regulator) rules for GMOs (Genetically Modified Organisms) as explained on the door sign. PC2 training courses are run twice yearly (emails will be sent in advance), make sure the trainee attends the next one. Food or drink for human consumption must never be stored in the Laboratory. If necessary to store food or drink for experimental purposes, clearly mark “Not for human consumption”. Before using most Equipment, Procedures and Hazardous Substances in the laboratory, you are required to complete a Training Agreement (and read the Risk Assessment). If you are unsure of which Equipment, Procedures or Hazardous Substances this applies to, ask your Supervisor or the Safety Officer. NEVER GO AHEAD IF YOU ARE UNSURE, ASK! Completed Risk Assessments are stored electronically on the V drive and the hard copies in the Red folder – Safety Folder 2. V:\Health, Safety and Environment\Risk Management MSDS‟s are also available electronically on Chemwatch: http://jr.chemwatch.net/chemffx/ Supervisor to show the basic functions of the Chemical Database on the V drive: V:\Resources\Chemical Lists\Chemical Database 14 When acquiring or disposing of chemicals a “Change to Chemical Database Notification” form must be filled in and placed in the appropriate place (specific to each laboratory). Forms can be found on the V drive: V:\OHSE\Labels, Signs & Templates\Templates\Change to Chemical Database Notification Supervisors are to highlight the importance of correct chemical storage. Show how to find correct storage on MSDS. Importance of only putting flammables in “intrinsically safe‟ fridge/freezers. Incorrect storage could result in a reaction producing an explosion, fire or emission of poisonous gasses. Supervisors to explain Waste Management procedures specific to the laboratory. Biological waste, sharps, chemical waste (no glass containers used), quarantine – AQIS guidelines for disposal of waste. Any other laboratory-specific procedures? Gas bottle management? Acid bath maintenance? 6.4 Training Agreements 6.4.1 Training for Equipment Any piece of equipment that is specific to your laboratory and requires training of new users should have a training agreement. Each piece of equipment must have a responsible person appointed and their name(s) posted on it. This person is responsible for: Training users – this should include: Familiarity with the machine, its controls and the requirements of the manufacturer’s handbook Operating rules and limitations Emergency shutdown procedures and incident reporting Record keeping, log books Other requirements related to chemical or biological safety Keeping Records of authorised users: Name, date trained and authorised Ensuring records are maintained Maintenance. All staff and students trained to use this piece of equipment must also read the Risk Assessment (complete a new one if not available, see section 12.2) and complete a Training Agreement in Safety Folder 2. 15 6.4.2 Training for Hazardous Substances The trainee should read the MSDS and direct any questions to their trainer. The trainer should explain any safety guidelines for using the Hazardous Substance, including PPE, usage conditions, spill procedure and waste disposal. After training, the trainee must know: Correct PPE to wear Where it can be used (i.e. open laboratory, fume hood) Decontamination/clean up procedures Disposal procedure Spill procedure First Aid (i.e. Skin, eye or respiratory contact) All staff and students trained to use this Hazardous Substance must also read the Risk Assessment (complete a new one if not available, see section 12.2) and complete a Training Agreement in Safety Folder 2. 6.5 Risk Assessments Risk assessments for all research projects that include procedures using any Laboratory Equipment, Processes or Substances that are a possible risk should be completed BEFORE commencement of any research work. These need to be regularly updated as projects progress. For guidelines on how to complete a risk assessment, see section 12.2 of this manual. 16 7 Inspections and Audits 7.1 In-house OHS Inspections OHS inspections should be completed twice per year by the designated laboratory member. This person’s name should be written in Safety Folder 1 on the “Laboratory Maintenance and Training Responsibilities” sheet in the appropriate place. An email will be sent informing of the date in which the inspection must be completed by. The designated person should work through the relevant sections of the Monash University Workplace Inspection Program found on the V drive: V:\OHSE\Inspections\workplace-inspection-program A summary of the inspection should then be completed using the template: V:\OHSE\Inspections\Inspection Worksheet Template This inspection summary must then be saved in the appropriate folder, using the Supervisor’s name as the file name. Summary folders are found at: V:\OHSE\Inspections If you have any difficulties completing the inspection or questions regarding the inspection, consult the Safety Officer. 7.2 External Audits 7.2.1 OHS External Audits Monash University arranges a team of OHS staff (from outside of the School of Biological Sciences) to perform an OHS Audit annually. The best way to prepare for this audit is to work through the relevant sections of the Monash University Workplace Inspection Program found on the V drive (as used for in-house audits): V:\OHSE\Inspections\workplace-inspection-program 7.2.2 Worksafe inspections Worksafe inspectors can come in and inspect at any time. An inspection checklist with information that all lab members should know is available on the V drive: V:\OHSE\Inspections\Worksafe Inspection Checklist 17 7.2.3 OGTR Inspections The Office of the Gene Technology Regulator (OGTR) Compliance & Investigation team usually perform inspections annually at an arranged time, however can also inspect anytime without warning. OGTR staff review practices with regards to the following areas: Transport, Storage and Disposal Notices, Notifications and Records People management On the day of an audit, OGTR staff may select ANY dealing from our OGTR database of current projects and ask researchers to show/explain: How the GMOs are used Where GMOs are stored How GMOs are disposed They will also be required to show records/provide details of: • Transport relating to GMOs • GMO records • Training records for Biosafety, OHS and laboratory inductions The OGTR undoubtedly also observe people’s practices and behaviour’s within the laboratories during their visit. Should you have any questions or concerns relating to an OGTR audit, please do not hesitate to contact the Research Compliance Office on 9905 5162. 7.2.4 Quarantine Inspections AQIS (Australian Quarantine and Inspection Service) usually inspect biannually on announced visits, however they may also inspect anytime unannounced. Groups should ensure they meet/follow the criteria for their QAP at all times. This criterion is available on the website: http://www.daff.gov.au/aqis/import/general-info/qap/qapcriteria If you have any further questions, see the Safety Officer. 7.2.5 Drugs and Poisons Audit Department of Human Services occasionally perform a Drugs and Poisons Audit. The School has a Poisons Control Plan (PCP) that outlines the requirements to comply with School permit conditions. It is available on the V drive: V:\Resources\Permits - AQIS, DSE, DPI, EA, Poisons\Poisons\Poisons Control Plan\Current 18 8 Electrical Testing 8.1 Residual Currency Devices (RCD’s) Residual Currency Devices (RCD’s) have been installed in laboratories. RCD’s monitor the residual current in a circuit to earth and cut off the power when the earth leakage reaches a predetermined current, generally 10, 30 or 100 milli amps. To comply with Australian Standards the RCD needs to be tested “regularly”. One RCD protects one circuit. Each RCD circuit is numbered. You will know if a circuit has been fitted with a RCD by the presence of an extra button between the two power switches. Not all equipment should run off RCD protected circuits. Equipment not recommended to be connected to RCD’s are items that may contain perishables such as refrigerators, freezers, etc. and protection devices requiring permanent power, as any interruption to supply due to tripping may defrost or render protection devices inoperable. Each section MUST test the RCD’s in their area in March and October each year and recorded in the table in the “Safety Equipment Testing” section of Safety Folder 1 – “Safety, Training and General Information”. RCD’s are tested with a simple “push button test‟. Before performing the test, remove or turn off any equipment from the circuit which may be affected by a temporary power loss. Take a lamp (or any other electrical device), plug it in to any point within the circuit and turn it on. Push the “test” button and observe the instant loss of power. Push the reset button, record the test in the “Safety Equipment Testing” section of Safety Folder 1 and move to the next circuit. If the power did not terminate as expected, report the fault to the Buildings and Infrastructure Officer. 8.2 Testing, Tagging and Repairing Electrical Equipment Testing and tagging of office and laboratory equipment is carried out yearly. A group email is sent to everyone in the department to inform them of when this will take place. Equipment which is not free to be tested (i.e. being used in an experiment), needs to be logged as a job and taken to the workshop when it is free. Electrical appliances brought in by contractors or from home for use on a Monash site are subject to the same testing and tagging procedure for appliances owned or leased by the University. Appliances should be tested and tagged prior to their use on university premises. While there is no requirement to test and tag personal laptops, staff and students are encouraged to have their laptops tested and tagged using an approved person or company. In both situations above the testing and tagging is the responsibility and expense of the owner. If the expense is to be covered by the Office or Laboratory where the item is to be used, a job can be logged using the relevant fund code and the item taken to the workshop (or an on -site job requested if the item is too large to be moved). 19 For further information, see OHS Information Sheet # 13 – Inspection, testing, tagging & repairing electrical equipment. http://www.monash.edu.au/ohs/topics/onfo-sheets/testing-tagging-repair.html 20 9 Testing Emergency Eye wash and Safety Showers 9.1 Testing Emergency Eye Wash To test the emergency eye wash, remove the rubber caps from and run water at full pressure for 20 seconds or until water runs clear. This should be repeated fortnightly. Record testing in the “Safety Equipment Testing” section of Safety Folder 1 – “Safety, Training and General Information”. 9.2 Testing Safety Showers Testing of Safety Showers does not need to be performed by laboratory members. Facilities and Services Staff test them twice per year. 21 10 Hazards and Risk Areas within the School The following section details items of special relevance to this School: Risk / Hazard Causes / Effects Management Comments Burns Excessive heat Hold the burnt area Chemicals Electricity under cold gentle Radiation running water. Seek medical advice. Do not apply lotions or break blisters Frost Bite -70 °C Freezer Liquid Nitrogen Contact First Aider Rewarm the area by body heat. Never rewarm with direct heat or rub the frostbitten area. Needle stick injuries Syringes with needles Do not recap needles Control bleeding with pressure bandage. Contact First Aider. Cuts, Lacerations Rubber Bulbs or Pi-Pumps with glass pipettes or Pasteur pipettes Avoid using glass As above pipettes where possible, especially if the graduations are faded. Use safety pipette fillers and or plastic pipettes UV Radiation, causing sunburn, skin cancers, eye damage UV lights in laminar flow cabinets, transilluminators, solar radiation Labs: UV safe Not all lab safety glasses, face shields glasses are UV safe and Perspex screens. Outdoors: Sunscreen, sun hat, sun glasses Contact lenses Chemicals and chemical vapours can get trapped under the eyelid Contact lenses should not be worn when working in the laboratory. Goggles or Prescription safety glasses Acrylamide Toxic and suspected If splashed on skin or Refer to MSDS. carcinogen. in eyes, immediately Wear safety glasses, wash with water and gloves call first aider. 22 Risk / Hazard Causes / Effects Chloroform Toxic by inhalation - If splashed on skin or drowsiness, nausea in eyes - immediately wash with water and and vomiting call first aider. Diethyl Pyrocarbonate Produces hazardous vapours and a suspected carcinogen In case of contact, Refer to MSDS. Use immediately flush is discouraged eyes or skin, remove contaminated clothing Ethidium Bromide Mutagen, irritating to mucous membranes and upper respiratory tract If splashed on skin or in eyes, immediately wash with water and call first aider. Refer to MSDS. Wear safety glasses, gloves and lab coat. Weigh out powder in fume hood. Phenol Poison and burns If splashed on skin or in eyes, immediately wash with water and call first aider. Refer to MSDS. Wear safety glasses, gloves and lab coat. Weigh out powder in fume hood. Pesticide & Herbicide Spraying Many health problems Use appropriate protective clothing. Protective clothing available from the Botany Experimental Area. Electrical Work Electrocution Any modifications to electrical equipment must be carried out by a qualified person. Zoonoses, including Illness and allergies Q-Fever, Lyssa Virus For females, (see Appendix 7) additional dangers during pregnancy Management Animal Care Precautions, appropriate vaccinations, specified hygiene routines. Comments Refer to MSDS. Wear safety glasses, gloves and lab coat. Use in fume hood Notify your supervisor if you are feeling ill or suspect you may be pregnant 10.1 Health and Safety Issue Resolution For clear, step-by-step directions on Health and Safety Issue Resolution for an immediate or non-immediate Health or Safety Hazard, see the flow charts presented in the below OHS document: http://www.monash.edu.au/ohs/topics/procedures/issue-resolution.pdf 23 11 Hazard & Incident Reporting & Investigation The University has an Incident Reporting Policy, whereby all accidents, incidents and hazards are reported and recorded. The reporting of these incidents is important to assist the continual effort made by the University to maintain a safe working environment. Corrective action may then be taken from the reports to prevent any recurrence of an injury or situation. The School asks all staff and students to be proactive in this. Please do not be hesitant about reporting an incident in case someone gets into trouble: instead, please think of it as a way of fixing a hazard or a problem BEFORE anyone gets into trouble! The report form can be downloaded from the OHS&E website at http://www.monash.edu.au/ohs/forms/index.html (Download: Hazard incident report form) All incidents and hazards should be reported to the Safety Officer as soon as practicable. They will assist with the processing of documents required to satisfy the University’s Incident Reporting System and Work Cover Act requirements. If the accident is serious enough to warrant first aid, the attending first aider can also assist in the completion of Incident Reports. Although the prime concern of safety officers and safety codes is the protection of persons, an additional function is the protection of scientific and structural assets. Accidents do not just happen: they are caused, and one of the main causes is poor housekeeping. So please keep your work area tidy and the floor uncluttered. 24 12 Risk Management 12.1 Responsibilities Monash University’s Victorian campuses are all covered by the Victorian OHS Act 1985 and its subordinate regulations and codes of practice. An essential part of all of this modern OHS legislation is the requirement for workplaces to undertake risk assessments on all of its activities which may impact the health and safety of the employees, visitors, contractors and students. All staff and students are expected to play a role in this. In particular: All staff and research students must undergo a formal induction process when they first join the School. All staff and research students must attend a training session in the Identification and Management of Risk. These sessions are run regularly and the dates are widely publicised throughout the School. Special sessions are run in February and July for commencing Honours students. All Honours and postgraduate students must complete a written risk assessment and ensure it is approved by your Supervisor before they begin work on their project. All staff involved in field work, especially in remote areas, must complete a written risk assessment and ensure it is approved by your Supervisor before undertaking their first field trip. Staff and students wishing to carry out overseas field work must seek special authorisation from the Head of School and comply with additional special requirements. 12.2 The Risk Assessment Process The risk assessment process at Monash University is outlined in Reference sheets developed by OHSE: http://www.monash.edu.au/ohs/forms/risk-management-program.pdf The School has developed templates for this purpose (on the V drive). V:\OHSE\Risk Assessments\ a How to- & Template These forms are designed to assist assessment teams to follow the standard risk assessment process of Identification, Assessment & Control for all of the major hazard groups that are present at Monash University. However, please also bear in mind that additional information may be needed to allow your Supervisor to determine whether you have adequately addressed all the risks associated with your project. The major hazard groups you will need to consider are: Manual Handling Hazards 25 Equipment & Process Hazards Chemical Exposure Hazards Biological Exposure Hazards Radiation Exposure Hazards Environmental Hazards By reviewing your work area and activities against each of the major hazard groups, a comprehensive assessment will be produced that incorporates all of the OHS legislative requirements and University standards. The reference sheets have been designed to allow assessment teams to quickly and comprehensively identify and assess the hazards in the workplace, rank them in terms of priority and provide guidance for the development of appropriate control measures. The greatest amount of time and energy should be spent on selecting an appropriate control measure and instigating it. One person from within the group should be responsible for following through and ensuring the controls are put in place by the work area or support services as appropriate. Completed Risk Assessment projects should be uploaded to the V drive (choose the appropriate folder): V:\OHSE\Risk Assessments 12.2.1 Helpful Hints Some completed assessments are available for reference on the V drive. Be sure to read and refer to existing Monash University and School Guidelines, Policies and Procedures. 26 13 Immunisation If you fall into one of the following categories, you should consult with the Safety Officer and OHSE Physician regarding your individual immunisation program: First aider Work with Animals Work with Human Subjects or Bodily Fluids The following are some of the standard immunisations that you may be advised to take: Tetanus Hepatitis B Q-Fever Tuberculosis Rabies Immunisations may also be available at the School’s cost to other staff or students who feel they may be exposed to potential infections through their work or studies. If you wish to discuss this possibility, please contact the Safety Officer. Also see: http://www.monash.edu/ohs/topics/procedures/immunisation.pdf 27 14 Safe Laboratory Practices The following rules apply at all times in all laboratories: No eating or drinking No smoking Appropriate closed-in footwear must be worn - bare feet or open toed sandals are not permitted Observe safety precautions - use fume cupboards, wear lab coats, gloves, safety glasses as necessary Never undertake any work unless you are aware of all known and possible hazards have adopted appropriate safety precautions Clean up all spills and breakages immediately Do not work in isolation - ensure a second person is within call Label all your solutions, samples etc. with exact contents, your name and the date. Unlabeled items will be thrown out Post warning signs for unusual hazards such as flammable materials, biohazards or other specific hazards Thoroughly wash and rinse all glassware as soon as you have finished with it do not let it accumulate on the sink. Replace in correct location in cupboards or drawers Keep your equipment etc. together and minimise your area of spread. Use equipment only for its designated purpose Leave laboratory equipment (microscopes etc.) free when you are not using them so that others may do so Wash skin areas which have come into contact with chemicals, irrespective of concentration Never adopt a casual attitude and always be conscious of potential hazards The following “housekeeping” practices should also be adhered to: Eliminate safety hazards by maintaining laboratory work areas in a good state of order Always keep tables, fume cupboards, floors, aisles and desks clear of unnecessary material Wipe down bench tops and other laboratory surfaces after each use with an appropriate cleaning agent or disinfectant Each laboratory will have a Safety Information and Training Agreement Folder. This folder contains relevant information on the day to day functioning of the lab - be sure to use it 14.1 What each Laboratory needs to do and have 1. Prepare a one-page Laboratory Induction program, which must be given to new staff and students that will be working in the lab 2. Conduct an actual induction to the workings of the laboratory 28 3. Maintain the information in the Laboratory Safety Information and Training Agreement Folder 4. Ensure new staff and students are trained in the use of chemicals and equipment, and that this is documented 5. Maintain the V: drive chemical register and MSDS file 6. Complete and file Risk Assessments for designated Hazardous Substances and all procedures 7. Ensure appropriate Personal Protective Equipment is available, maintained and used 8. Understand the requirements of the Regulatory bodies that govern activities in the laboratory (i.e. AQIS, OGTR etc.) 9. Label all solutions with: Name and Concentration of Solution Date made Lab code as per coloured labels The owner’s name 10. Maintain good housekeeping standards at all times 14.2 Personal Protective Equipment (PPE) Enclosed footwear must be worn in this area Personal Protective Equipment is an important part of your work environment. If you have difficulty locating the appropriate pieces of equipment, please speak with the Safety Officer. 14.2.1 Footwear Shoes must be worn at all times in these buildings. Shoes with enclosed toes are mandatory for all student practical classes and any workshop or laboratory work involving any chemical/hazards. Thongs or sandals are not acceptable footwear in any laboratory. 14.2.2 Protective Clothing / Lab Coats Lab coats MUST be worn at all times when working in a laboratory classified as PC1 or PC2, or in a Radiation Laboratory. It is also strongly recommended that fastened lab coats are worn during any laboratory procedure. Providing your supervisor (if appropriate) agrees, a second-hand lab coat may be available from the Store (if there is not one in your size, the purchase price of a new one is borne by your section). The laundering of lab coats can be arranged through the Faculty of Science Store. The cost of this is charged to your section. 29 14.2.3 Eye Protection “An estimated 90% of eye injuries can be prevented with the proper use of protective eye wear.” Safety glasses/goggles or a face shield MUST be worn whenever there is a potential hazard source such as chemicals, dust, flying fragments. Each laboratory has safety glasses available for general use. If these do not fit you or are otherwise unsuitable, please consult the Technician in charge of your area or the Safety Officer, who has a catalogue available and may be able to recommend suitable glasses. 14.2.4 Hearing Protection There are recommended exposure levels (dBA or decibels /hour) for the workplace environment. If you are working with “noisy” equipment, regardless of the exposure time, you should wear hearing protection. If you believe you are at risk from exposure to high noise levels, consult with the Safety Officer who will arrange with OHS&E for further tests to be carried out. 14.2.5 Hand Protection Protective gloves should be worn when working in a laboratory when handling Hazardous Substances or Dangerous Goods. It is also strongly recommended that suitable gloves are worn during any laboratory procedure. However, it is important that you select gloves on the basis of the material being handled. For example: PVC protects against mild corrosives and irritants Latex provides only light protection against irritants Neoprene is ideal for working with solvents, oils or mild corrosive materials Cotton absorbs perspiration and helps keep objects clean For more information, consult the Ansell Chemical Resistance Guide at http://ansellpro.com/download/Ansell_8thEditionChemicalResistanceGuide.pdf 14.2.6 Respirators Staff and students working in close contact with insects should be aware of potential allergies. Precautionary measures should be discussed with the Safety Officer and/or OHSE. The school owns a respirator that should be used when there is a risk identified. Individual/personal face pieces for this may be purchased as required, at a cost to your section. The Faculty of Science Workshop (on the ground floor of Building 17) has a well-equipped wood shop with high-quality dust extraction system. Subject to the approval of the Workshop Manager, staff and students are welcome to make use of this for any jobs which generate large amounts of dust. 30 Disposable dust masks can be purchased through the Science Faculty Store for any smaller dusty job. Respirators must be cleaned with Hibitane or an equivalent disinfectant between uses. 14.3 Ergonomics in your Work Space Having an ergonomic work space is important to lessen the likelihood of workplace injury and provide an environment where you are at your most productive. 14.3.1 Computer/Office Work Stations The Monash OHS team created “Computer User Guidelines‟ which provides helpful hints for setting up your desk area, computer and chair. It also contains exercises for office based workers. See section 21.5 for “Screen Based Equipment Guidelines and Exercises‟ and see: www.monash.edu.au/ohs/topics/guidelines/computer-user.pdf 14.3.2 Laboratory Such principles are fundamentally similar when working in the laboratory. Tasks which require frequent awkward posture are the most common causes of Repetitive Strain Injury (RSI). Pipetting, microscope work and hood work are generally the most repetitive tasks in laboratory work and are therefore likely to be associated with reported injuries. 14.3.3 Pipetting The manual plunger-operated pipette can cause RSI and other muscle strains. Some tips when choosing and using the pipette: Before ordering a particular pipette, try to find one to borrow to see if the size, weight, shape and position of mechanisms are comfortable for you Replace a manual plunge-operated pipette with an electronic multi-channel (or single channel) pipette whenever possible If a manual plunge-operated pipette needs to be used; take regular breaks and try to swap hands or fingers using the plunger Try not to rotate your wrist while pipetting and hold with a relaxed grip using minimal force Position your containers/tubes/waste in such a way that you do not have to overreach, twist or bend repetitively to complete the task 31 14.3.4 Microscope Operating a microscope for long hours can cause strains in the neck, shoulders, eyes, back arms and wrists. The likelihood of such strains occurring can be greatly reduced by: Your microscope must be set up at a height Position the microscope towards the edge of the bench allowing adequate forearm support. Ensure there is legroom directly under the microscope Adjust the height of your microscope so that when you are sitting straight backed in your chair and looking straight ahead, the eye pieces the same height as your eyes and angled 30-45˚ below your line of sight. If the height of your microscope is not adjustable, stack blocks of solid material under the scope to achieve the desired height Take regular breaks. Get up and move around every 30-60 minutes. Set a timer to remind yourself! Rest your eyes. Focus on something distant at least every 15 minutes. Cup your hands gently over your eyes to rest them from the light for a minute. Do not rub your eyes Check the surrounding environment for sources of excessive glare/reflection and reduce them accordingly. Excessive glare/reflection results in using more illumination when using the microscope and can cause eye strain 14.3.5 Laboratory Hoods The forward head and extended arm positions can cause neck, shoulder, back and arm injuries. To minimise risk of strains: Position materials in a way that avoids excessive reaching (without compromising the containment of the cabinet) If sitting, use a fully adjustable chair and adjust it to a supportive position. Use a foot rest (not the ring of the chair) to provide a stable support when leaning forward to work If standing, use an anti-fatigue mat and wear supportive shoes Keep the viewing window clean and un-obstructed Take frequent breaks 14.3.6 Other Laboratory Tasks Although laboratory tasks vary, keep the following basic guidelines in mind to reduce your risk of injury: Set up your work space ergonomically specific to your needs (i.e. posture, support) Try to reduce twisting and awkward positions by strategically placing materials 32 Take frequent breaks If you need any further information or would like to arrange a personal ergonomic assessment, contact your local OHSE consultant or the general OHS branch. 14.4 Unattended Experiments Consideration must be given to alternatives to running equipment, ovens and experiments overnight based on risk. A manually resetting over-temperature cut-off switch, which is set to operate at a temperature slightly higher than the upper limit of the controlled temperature, must be fitted to all thermostatically controlled equipment. Electrical equipment left on after hours should carry a 'Do not switch off' notice, giving the name of the person leaving the equipment and contact details. Each lab has laminated “experiment in progress” signs to use which are located in the front cover of Safety Folder 1 “Safety, Training and General Information”. More can be printed from the V drive: V:\OHSE\Labels, Signs & Record Sheets\Signs\Experiment in Progress The experiment should also be entered into a book at a central point in the unit/entity by 4 pm of the day of the experiment, listing the details described in section 9.2.1 of this procedure. A representative of the unit/entity should then notify Security & Traffic, in writing, before 5 pm of all unattended experiments. Before initial use and on redesign of apparatus to be used in unattended experiments, staff and students must get the apparatus checked and the card initialed by their supervisor or an experienced person before leaving for the day. The person responsible for the experiment should attend when the apparatus is started. Frequent checking of the apparatus and/or experiment should then be carried out for a sufficient period to ensure that the apparatus is working well and to identify and rectify any problems. http://www.monash.edu.au/ohs/topics/index.html (Select “After hours work”) 33 15 Chemical Safety 15.1 Definitions and Legislative Requirements The management of Chemicals is regulated by three legislatives acts: Dangerous Goods Hazardous Substances Controlled Substances (Drugs and Poisons) These Acts all operate independently of one another and so chemicals may fall into all of these categories, or one or two, or none. For further information on the topics below, see: www.monash.edu.au/ohs/management-system/using-chemicals.pdf 15.1.1 Dangerous Goods The Dangerous Goods Act deals with the physical properties of the chemical, such as whether it is flammable, corrosive, explosive, etc. All goods which fall within this Act are divided into Chemical Classes, as follows: Class number Class name 2 Gases 3 Flammable Liquids 4.1 Flammable Solids 4.2 Substances Liable to Spontaneous Combustion 4.3 Substances when wet with water evolve flammable gases or spontaneous ignite 5 Oxidising substances and Organic Peroxides 6.1 Poisonous and Toxic Substances 7 Radioactive Material 8 Corrosive substances 9 Miscellaneous dangerous goods and articles 15.1.2 Hazardous Substances The Hazardous Substances Regulations deal with the health effects of a chemical. A hazardous substance is defined as a substance which may be hazardous to health due to either its acute toxicity or the potential for chronic adverse health effects. A defined 34 hazardous substance is classified by “R“ (risk) phrases, such as “R34: Causes burns” or “R36/38: Irritating to eyes and skin.” Hazardous substances may also be classified by use of “S” or safety phrases such as “S15: Keep away from heat” or “S7/8: Keep container tightly closed and dry.” These phrases may also apply to chemicals not defined as Hazardous Substances. Occupational Health and Safety (Hazardous Substances) Regulations 1999 came into effect 1st June 1999. These regulations are additional to existing legislation for the safe management of chemicals, Dangerous Goods and Controlled Substances. They include four main requirements: Development and maintenance of a register of hazardous substances used and copies of Material Safety Data Sheets for these substances must be held. Assessment is required for all hazardous substances and must take into account the method of use. Appropriate risk controls must be implemented based on the outcome of the risk assessment. Appropriate training must be provided to all staff and students who use hazardous substances. Licensing and notification is required for use of a specified list of carcinogens. Further information on the above can be found in the Safety Information and Training Agreement Folder in your lab. 15.1.3 Controlled Substances (Drugs and Poisons) This category covers a wide range of chemicals, including but not limited to those which: Are prescription-only or pharmacy-only medicines Are, or may be used in the manufacture of, legal or illegal drugs of addiction Are used for medical or veterinary purposes Are used as agricultural, industrial or household herbicides or pesticides The School holds a Poisons Permit which allows us to purchase some (but not all) subclasses of controlled substances. 15.1.4 Purchase and Storage of Scheduled Poisons People commonly use the term “poison‟ to describe many different chemicals. These may be dangerous goods, hazardous substances and/or scheduled poisons. For a list of categories and the corresponding storage method, see: www.monash.edu.au/ohs/topics/poisons.pdf 15.1.5 Working with Scheduled Carcinogens Carcinogenic substances are hazardous substances that can cause cancer. Use of such substances should be eliminated whenever possible, and/or a safer alternative sought. A carcinogenic substance must not be ordered without the Supervisor’s advice and specific approval. Following this, a risk assessment must be complete and approved by the Supervisor PRIOR TO WORK. 35 Two schedules of carcinogenic substances are restricted under part 4.2 of the Occupational Health and Safety Regulations 2007. Two schedules of carcinogenic substances are restricted under part 4.2 of the Occupational Health and Safety Regulations 2007. Schedule 1 carcinogenic substances The use of Schedule 1 carcinogenic substances is only permitted in laboratories after a license is obtained from WorkSafe Victoria. Use of these substances in workplaces other than laboratories is prohibited. 2-Acetylaminofluorene Aflatoxins 4-Aminodiphenyl Benzidine and its salts Bis(chloromethyl) ether Chloromethyl methyl ether (technical grade) 4-Dimethylaminoazobenzene 2-Naphthylamine and its salts 4-Nitrodiphenyl Schedule 2 carcinogenic substances The use of Schedule 2 carcinogenic substances is only permitted in workplaces (including laboratories) after a license is obtained from WorkSafe Victoria. Schedule 2 carcinogenic substances are: Acrylonitrile Benzene – when used as a feedstock containing more than 50% benzene by volume 3,3'-Dichlorobenzidine and its salts Diethyl sulfate Dimethyl sulfate Ethylene dibromide – when used as a fumigant 4,4'-Methylene bis(2-chloroaniline) 2-Propiolactone o-Toluidine and o-Toluidine hydrochloride Vinyl chloride monomer Further information can be found at: http://www.worksafe.vic.gov.au 36 15.2 Maintenance and Requirement of Chemical lists and Material Safety Data Sheets The University is required by law to comply with strict requirements relating to the storage, handling and documentation of chemicals, and is regularly audited by WorkCover inspectors. The co-operation of all members of the School is essential to ensure that requirements are met. All details relating to the hazardous nature and dangerous goods class of any chemical can be found on the Material Safety Data Sheet (MSDS) for that chemical. The School is required to maintain copies of the MSDS’s for all chemicals held in the School, whether or not they fall under any of the relevant legislation. The MSDS’s for chemicals in each laboratory in the school are held available on the V drive: v:\Resources\Chemical Lists\MSDS A hard copy of a Mini MSDS’s for each chemical is available: 17-2 corridor (opposite lifts) 18-107 Utilities Room The School is also required to maintain a list of all chemicals held within the School, specifying the quantities of each, the Dangerous Goods class, and other relevant information. If you bring a new chemical into any laboratory (whether borrowed from another laboratory or obtained from outside the School) then you MUST follow the procedures below to ensure that an MSDS for it is placed on file, and that it is recorded in the Chemicals list. 15.2.1 School Responsibilities As users of chemicals we are required by law to: Maintain a register of chemicals held in the School Obtain a brand specific MSDS for the chemical (no older than 5 yrs) Understand and follow the storage, handling and disposal requirements of the chemical For all designated hazardous substances, complete a written risk assessment Train new staff and students in the use of chemicals and document that this training has taken place In 2004, the School invested significant resources to ensure all laboratory chemical lists and MSDS files were up to date. Each laboratory must now ensure chemical lists and MSDS files are maintained to comply with the legislative requirements. The School cannot afford to audit chemicals every year! In order to identify what chemicals are already in the database, all chemicals in each lab will be labeled, with a coloured label and lab name, identifying the lab that owns the chemical. The School has a central database for chemicals, which is held on the V drive. Each lab must have a designated person who is responsible for maintaining the list. An instruction sheet for using the chemical data base can also be found on the V drive: 37 V:\Resources\Chemical Lists\Chemical Database Also available in the V-drive folder will be electronic copies of some common MSDS’s. MSDS’s are available from the Faculty Store for any chemicals purchased from there. V:\Resources\Chemical Lists\MSDS 15.2.2 What does EVERYONE need to do? When acquiring or disposing of chemicals by any mechanism, all members of the School MUST: Complete the notification form and leave in the designated tray in the laboratory, so that the designated person in their laboratory can update the Chemicals List and label the chemical. If the MSDS is delivered with the chemical, attach it to the Notification form so that the designated person can place it on file. Non-compliance is a very serious issue, and any staff member or student not taking reasonable steps to comply with the requirements set out will face disciplinary action. “Change Chemical Database Notification” sheets can be found at: V:/OHSE/ChemicalSafety/Guidelines/ Maintenance and Requirement of Chemical Lists and Material Safety Data Sheets 15.3 Storage Limits for Dangerous Goods in Laboratories These limits have been set up by OHSE to provide guidance on the maximum amount of dangerous goods that are to be kept in laboratories at Monash University. The limits have been set to achieve appropriate storage in accordance with the Dangerous Goods (Storage and Handling) Regulations 1989. Three storage categories have been designated - Low, Medium and High. The categories are based on mass/volume of a substance to assist in the classification of laboratory storage. See table over page entitled “Storing Dangerous Goods in Laboratories, Studios & Workshops”. It must always be remembered that these limits are meant to be upper limits and if it is possible to keep less than the amount specified this is desirable. 15.3.1 Special Items There are some items, which will need additional storage restrictions due to their hazardous nature and/or due to additional regulatory requirements. Advice is to be sought from the MSDS and Safety Officer on the storage of the following items: Scheduled Carcinogens Acrylonitrile Cyanides 38 Scheduled Poisons (as per Drugs and Poisons Regulations) Radioactive isotopes Perchloric Acid and its etchant mixtures 15.3.2 Storage of Dangerous Goods in Refrigerators/Coldrooms Flammable chemicals in need of refrigeration must be stored only in intrinsically safe (i.e. spark-free) appliances. For further information see pages 10-11 of the School Guide V:/Resourses/Induction Manuals/SchoolGuide 15.3.3 Gas Cylinders in the Laboratory Cylinders must only be kept in laboratories when they are in regular use. Do not keep spare cylinders “just in case”. Cylinders should be kept in specially constructed dedicated cages or cupboards wherever possible. Whether in a cage/cupboard or in the lab itself, all cylinders must be securely anchored at all times, using appropriate chains or belts fitted to clamps on benches or walls. On-the-spot fines of up to $3000 can be incurred if Worksafe Inspectors see an unchained cylinder. The correct regulators or valves must be fitted to cylinders. Obey the correct opening sequence of the valves, and if you are not sure what this is ask the Safety Officer. Make sure that valves are tightly shut after use. Keep oxygen away from flammable solvents. When moving a gas cylinder always ensure that it is carried on a suitable gas cylinder trolley, preferably one of the three-wheeled yellow trolleys available from the Faculty Store, and that the regulator is removed. For further information see: “Guidelines for Gas Cylinder Safety” V:/OHSE/ChemicalSafety/Guidelines/GasCylinderSafety 39 For an updated version of the above table see: www.monash.edu.au/ohs/topics/dangerous-goods-storage.pdf 40 15.4 Chemical Waste Disposal Procedures 15.4.1 User Responsibility Environmental legislation relevant to activities at Monash is associated with land, air and water quality as well as waste disposal. Under this legislation Monash has the following broad environmental responsibilities: Do not change or harm the environment Do not cause pollution or allow potential pollution to occur Minimise waste Adhere to the Legislation and Regulations from Federal, State and Local governments Waste from laboratories, called Prescribed Waste, includes biohazards, radioactive waste and chemicals which must be disposed of according to procedures provided by the university. All Trade Waste discharged into the sewer must comply with prescribed quality criteria which require the exclusion of laboratory substances. During all waste disposal procedures, it is important to prevent incidental contact by others (e.g. cleaners emptying bins or plumbers maintaining pipes). Correct waste management (hazardous and non-hazardous) involves a structured program to ensure that any wastes generated are correctly identified in terms of their potential hazard to the environment and any staff handling them. It also ensures wastes are correctly labeled, contained to ensure that they cannot spill, stored in a manner to prevent off-site migration of wastes and non-authorised person’s access, transported according to legislation and disposed of in accord with best-practice environmental guidelines. 15.4.2 Disposal of Chemical Waste Log the Waste to be Collected Dangerous Goods and Hazardous Substances are disposed of through the Faculty of Science Store. Collections are made every 2nd and 4th Tuesday of the month (there are no collections in January). Waste must be logged on the system before 12pm the Friday prior and taken to the store Monday afternoon: http://cfapps.sci.monash.edu/store/index.cfm Log in and select: Order Stock Categories Laboratory Waste This link also provides list of each classes, units of volume accepted and price for disposal. Prepare the Waste It is critical that you select a suitable container for the waste and the label accurately reflects the contents of the container. Plastic containers are the preferred option (due to 41 the potential hazard of glass containers rupturing) unless there is a significant incompatibility issue. Waste labels must contain the following details: • Name of generator and contact details: department and contact number • Description of contents of container (including estimates of concentrations where possible) • Dangerous goods class and coloured diamond sticker (a photocopied version is not acceptable) • Date of generation Templates for waste labels can be found on the V drive: V:\OHSE\Labels, Signs & Record Sheets\Chemical waste labels It is the laboratory’s responsibility to purchase label paper and print the labels when needed. Important Points Check that waste can be disposed of BEFORE producing it Dilution is NOT an acceptable means of overcoming appropriate disposal Do not fill containers more than ¾ full Keep containers closed when not in use Segregate incompatible waste Spill equipment should always be on hand For further information on the above points, see: www.sci.monash.edu/safety/docs/waste-disposal-guidelines.pdf 15.5 Spill Management Procedures When a chemical is spilt, it must be cleaned up carefully and quickly. All staff and students must ensure that they are familiar with the spill containment and clean-up procedures for all chemicals with which they are working. This information is detailed on the MSDS and also summarised on the chemicals list for your laboratory. Consult the MSDS BEFORE you start using the chemical - DO NOT wait for a spill to occur, as prompt action may be necessary to contain fumes or other hazards. If you are not sure of the correct disposal or spill clean-up procedure for any spilt chemical, if you are not sure what the spill consists of, or if there is anything else you are unsure about, consult the Safety Officer BEFORE taking any other action. 42 15.5.1 Spill Kits There are a number of spill kits available in the School: Small briefcase-sized kits in green plastic cases are located at sign-posted locations throughout the School. Larger high-volume kits in orange wheelie-bins are located near the main fair panel in each building. Please be sure to note the location of the closest kit to your lab, and make sure you know how to use it. Specialised kits are available for formaldehyde and radioisotopes. Safety goggles, gloves and boots are essential safety equipment to be worn when cleaning up a hazardous chemical spill. Any spill must be reported to the laboratory supervisor, the Safety Officer or the School Manager. Depending on the size and type of spill, Breathing Apparatus (BA) may be required please contact a trained BA user. 15.5.2 Mercury Spills The use of mercury thermometers should be strongly avoided because of the vulnerability to breakage. However, we have not banned totally the use of mercury thermometers in this School, because of their greater accuracy and sensitivity. Please assess your needs at the outset of the work and be aware of the toxic nature of mercury and take great care if mercury thermometers have to be used. Wherever possible, spirit thermometers should be used in preference. If a mercury spill occurs, the laboratory must be evacuated without delay and the door locked and signposted to prevent unauthorised entry. DO NOT attempt to clean up the spill unless you have been specifically trained to do so, including use of the Breathing Apparatus (BA) The person responsible for the spill should: Notify all other persons working in the laboratory that a mercury spill has occurred. Switch on any fume cupboards or ventilation systems in the room, and leave on whilst the room is in the evacuated state. Contact the Safety Officer. The person who does the clean-up must wear Breathing Apparatus (BA) and be trained in its use and a second person who is also trained in the use of BA should be standing by with a spare BA set ready to assist should the need arise. Rubber gloves must be worn during all clean-up processes. The suspected spill area should be sprinkled with zinc dust or the absorbent powder provided in the recommended spill kit. All mercury-contaminated waste must be secured in a plastic screw-top container in order that no vapour will leak out. The waste must be disposed of via a waste disposal contractor, as chemical waste. Under no circumstances should it be put in the ordinary garbage, radioactive or biological waste. Place in waste container and label clearly, all waste is disposed of via Chemsal. If a thermometer is broken in a full water bath, the water will be contaminated, so it must not be tipped down the sink. Water should be poured into a suitable waste container and sent to Chemsal to be discarded with other hazardous chemicals. If possible, clean up in 43 a fume hood to prevent inhalation of vapour. Prior to re-opening the laboratory the atmosphere in the vicinity of the spill site should be checked for contamination using an appropriate monitor. The OHS&E Branch can perform this function at the conclusion of the clean-up. Calls should be directed to Margaret Rendell (ext. 51016) or Richard Connelly (ext. 51034). 44 15.5.3 Chemical Spill Response Procedure Does the spill require evacuation? You will know from completing the Risk Assessment and reading the MSDS before using the Hazardous substance. Action depends on the chemical nature of the substance, the volume and location of the spill. Yes No Chemical Hazard spills requiring evacuation: 1. If safe to do so ensure the immediate safety of anyone within the vicinity of the spill. Ring 333 or use Red Emergency phone – You will need to give them your exact location Notify Supervisor or Safety Officer and OHS – 51016 2. Evacuate – as directed by the emergency personnel to the designated assembly point. 3. Clean up – Do not re-enter the area until it has been decontaminated by personnel train specifically in chemical safety. Chemical Hazard spills NOT requiring evacuation 1. SAFETY FIRST – Before attempting to clean up the spill, identify the spill to confirm appropriate Personal Protective Equipment (PPE) and clean up method. Refer to labels, MSDS‟, placards and supervisor/s. Wearing appropriate PPE: If the spill is flammable: remove sources of ignition. Caution: if lights or power points are "on" do not switch them “off" as this could cause sparks. If breathing difficulties are involved- isolate the area and contact the Safety Officer or somebody trained in using the Emergency Compressed Air Breathing Apparatus (See section 2.3 of Safety Manual). 2. Isolate the spill area (barricade tape and fold out danger signs supplied in larger spill kits). 3. Notify proper authorities (Supervisor, Safety Officer). 4. Prevent the spill from spreading. SELECT CORRECT AGENT FROM SPILL KITS: (located near laboratories for easy access) o Green kits o Big Yellow “wheelie” building Spill kits NOTE: Ensure that spill cannot enter drains by blocking potential pathway to drain with the clean-up agent. 5. Treat the spill. - Contain spill by pouring agent from bag or bucket around the perimeter - Cover spill by applying agent inward until all liquid is covered - Carefully mix agent 6. Remove contaminated materials - Make sure the contaminated material is sealed in a suitable container, correctly labeled and disposed of as waste via the Faculty of Science Store. - You may need to ask your supervisor/Safety Officer for assistance 45 15.6 Weighing Toxic/Hazardous Substances When weighing any substance for the first time, consult the MSDS to review its physical and hazardous properties. This will assist you in choosing suitable personal protective equipment, a safe weighing environment (Fume hood may be necessary if fumes or airborne particles are toxic/hazardous) and be aware of the spill cleanup procedure. If you have any questions after reading the MSDS, consult your supervisor (or the Safety Officer if your Supervisor is unable to answer your questions). 46 16 Biosafety Work involving recombinant DNA may be undertaken only in registered PC1 or PC2 laboratories in accordance with the “Guidelines for small scale genetic manipulation work” (January, 1993) issued by the Office of the Gene Technology Regulator (OGTR). A copy of the Guidelines is available from the Biosafety Officer, Dr Richard Burke. Further information can be obtained from Research, Grants and Ethics Branch or http://www.monash.edu.au/researchoffice/biosafety/ 16.1 Operating Procedures 16.1.1 PC1 and PC2 Laboratories The following procedures must be followed at all times in PC1 and PC2 laboratories: Laboratory doors must be closed when work is in progress. Laboratory coats or gowns must be worn at all times, and removed before leaving the laboratory. Hands must be washed with soap and warm water when leaving the laboratory and after handling cultures. All microbiological waste must be sterilised before disposal. Equipment used for handling cultures or contaminated material used which is not readily steam sterilised must be disinfected after use. A suitable disinfectant for glassware is a 5% sodium hypochlorite solution (for at least 30 minutes). The sodium hypochlorite solution must be made up fresh daily, and items must be soaked for 30 minutes. Work benches and surfaces must be regularly decontaminated with a disinfectant solution Material to be taken from the laboratory must be carried in a closed, unbreakable outer container. All technical procedures must be performed in a way that minimises the creation of aerosols. All work done in the PC2 laboratory must follow PC2 procedures whether or not genetic manipulation work is involved. No one should enter a PC2 laboratory for servicing of equipment or any other maintenance without the proper approval of the senior investigator in charge of the facility. The work areas must be disinfected before any such maintenance is carried out. All Biological Safety Cabinets must be tested and certified annually Refrigerators and Freezers should be labeled with a biohazard sticker if they contain biological material 47 16.1.2 Bacteriological Work The necks of culture flasks or bottles and sterile solutions should be flamed immediately after removal and before replacement of the tops. All cultures should be labeled clearly with the name of the organism and the user name. Cultures no longer required should be autoclaved as soon as practicable. Pipettes used for transfer of micro-organisms must be plugged with cotton wool and discarded into sodium hypochlorite immediately after use. Subsequently, plugs removed, then rinsed clean. Cotton wool plugs, should be removed from the pipettes and discarded appropriately, not put down the sink. Working with Pressurised Microbial Cultures – Syringe filters and French Press Safety glasses or face shields must be worn at all times when subjecting cultures to elevated pressures during filtration processes or the operation of the French press. If it is necessary to filter cultures to obtain sterile supernatants, the cultures should first be subjected to appropriate centrifugation in order to pellet and remove most of the bacterial cells. The supernatant should then be decanted, after which it can be filtered. If pre-filtration pelleting is not an option, then the filtration procedure MUST be carried out in a Class II Biological Safety Cabinet. Safety glasses or face shields must still be worn. 16.1.3 Class II – Biological Safety Cabinets Cabinets should not be used for storage. Ensure work area is clean by wiping down with 70% ethanol, a soluble phenol (do not use hypochlorite solution as it will eventually corrode the stainless steel). Plastic backed, absorbent sheeting (or trays) may be laid on work surface to facilitate clean up between procedures. Allow cabinet to run for at least 5 minutes to purge work area of contaminates. Plan procedure so as to place all required material and equipment in close proximity to cabinet. Check that UV lamp is off. Place material and equipment in cabinet. Remember to place work in the center of the work surface (items at the rear or front of the grille can impair air barrier performance). Vacuum services connected to the cabinet should incorporate an in-line microbiological filter to prevent the evacuation of infectious aerosols (0.2 µm recommended). Special care must be taken when using a Bunsen burner in proximity to solvents (ethanol etc.) in cabinets due to the fire risk. Avoid unnecessary removal of hands or material from the work area. 48 At the completion of work: Any equipment removed from cabinet should be wiped over with a suitable disinfectant. Wipe down the work area with disinfectant. Allow cabinet to run for at least a further 5 minutes. Switch on UV lamp (15 minutes before and after use is considered sufficient). Waste must be bagged and disposed in the correct manner. 16.2 Biological Hazards and Spills Spilt bacterial cultures require particular attention so that appropriate chemical (5% sodium hypochlorite) disinfection can be carried out as quickly as possible. All accidents and spills must be reported immediately to your supervisor, the Biosafety Officer or the Safety Officer. 16.2.1 Spills Outside a Biological Safety Cabinet If there is a spill of biological material outside Biosafety cabinet: Leave the room immediately, and ensure that everyone else in the room does the same. Close the door and place “DO NOT ENTER” and “BIOHAZARD” signs Remove all clothing that is grossly contaminated and place in a biohazard bag. If shoes may be contaminated, remove and place in a separate biohazard bag Wash hands and face and put on a clean laboratory gown Warn others of the spill and to keep out of the area Notify area supervisor or have someone do it for you Stay out of the spillage area for at least 30 minutes A full body shower is recommended Consult the Biosafety Officer before commencing clean up 16.2.2 Cleaning Up a Biological Spill The emergency response for an accidental spillage of biohazardous material in the laboratory will depend upon the hazard of the material and the volume. A minimally hazardous material that is spilled without generating significant aerosol may be cleaned up with a paper towel soaked with an effective decontaminating agent. A spill of a large volume of infectious material with the generation of aerosols will require cleanup personnel wearing protective clothing and respiratory protection. Other types of spills that may generate hazardous aerosols includes: spills within centrifuges and the release of biohazardous materials within refrigerators, incubators, or shaker baths. The same principles discussed apply. General procedures for clean-up are as follows, but you should seek advice from the Safety Officer or Biosafety Officer regarding your particular spill before taking action: Wait a minimum of 30 minutes before re-entering the room. Clean up personnel should wear gowns, rubber boots and rubber gloves. Respiratory protection may be required. 49 The team should preferably consist of three people, one to stand back and observe and direct the other two to contaminated areas. Before commencing, observe the spill area to determine the area of contamination Hypochlorite (5000mg/l chlorine) disinfectant is appropriate for many micro-organisms. Pour the solution carefully around the outside of the spill and allow to flow into the spill. Lay paper towels wet with disinfectant over the spill. Wait 20-30 minutes to allow disinfectant to act. Transfer all materials from the spill area to a metal pan for removal. Note: do not autoclave hypochlorite solutions. Wash and mop the adjacent areas, as well as the spill area itself, with fresh disinfectant solution. The decontamination team should wash their boots and gloves before removing them and leaving the area. Any other protective clothing should be autoclaved or decontaminated. If a Person is contaminated: Assess if it is safe to assist the affected person. The affected person should be removed from the contaminated area, if it is safe to do so. Initially, physical removal of the microorganism can be carried out by flushing or flooding contaminated skin with copious amounts of water, preferably using a safety shower or appropriate hand-held nozzle. This process will significantly dilute or remove the contamination agent. Contaminated clothing should also be treated appropriately, either by treating with sodium hypochlorite solution, an alcohol-based disinfectant or full steam sterilisation. If it is deemed necessary, this step can be followed by decontamination of skin surfaces (excluding the eyes and open wounds) using an appropriate alcohol-based wash or using soap and water. Containment of the wash effluent may be necessary if decontamination is necessary prior to it being discharged into the sewer. Biological Spills in Centrifuges Any spill in a centrifuge rotor must be immediately mopped up and decontaminated with 70% Ethanol (NOT Sodium Hypochlorite). Major spills must be reported so that the centrifuge can be inspected for potential damage to the drive assembly. 16.3 PC2 and Quarantine Glasshouses The School has a number of glasshouse bays which are classified by the OGTR as PC2 and/or registered by AQIS for growth of quarantine plants. Special requirements apply to these as detailed below, in addition to the usual rules governing safe use of School facilities. A separate manual exists for the quarantine bays, and all users must ensure they are familiar with it and comply with its requirements. 50 16.3.1 Operating Procedures All rules applying to PC2 laboratories and/or quarantine areas (as appropriate) apply in these glasshouse bays. Users must also comply with the following requirements: The glasshouses must be inspected regularly to ensure that containment features are intact, and any crack, chips or leaks noted must be reported to the Plant Resources Officer immediately. All doors to the plant house must be locked for the duration of the work except for those periods when personnel are actually working inside it. Hands must be washed with soap and water before leaving the plant house. Only authorised persons are to enter these glasshouse bays. All such persons must be trained to follow normal plant house routines as well as these operating procedures. All plants in PC2 bays must be treated as containing recombinant DNA. If quarantine plants are being grown, all plants in the quarantine bay must be treated as being quarantinable. Operations which may generate aerosols must be done in a biological safety cabinet as specified for PC2 containment. Plants and tissues taken into or out of the PC2 or quarantine bays must be carried in closed containers. Waste plants, tissues, soil, soil substitutes and the containers must be sterilised. The floors should be swept/vacuumed and mopped regularly Living plants or tissues must not be taken from the registered bay unless to a PC2 laboratory or Quarantine Approved Premise (as appropriate). Where the work permits, plants should be sprayed regularly with a systemic insecticide. The experimental materials must be inspected regularly for signs of arthropod infestation. Users of the PC2 should wear substantial footwear and take precautions to avoid transferring transgenic seed or plant material in their clothing or footwear when leaving the facility. Users of the quarantine facility must either walk through the disinfectant footbath when leaving or wear protective footwear whenever inside the facility and leave that footwear behind when they leave. 16.3.2 Containment Precautions If transgenic plant material is accidentally dropped or dispersed within the glasshouse bay, all material (including seeds) should be carefully collected into a covered, sealable container and autoclaved. A vacuum cleaner and portable electric vacuum brush should held in the facility for this purpose. The area of the spill should then be swabbed carefully with 5% sodium hypochlorite. 51 16.4 Handling and Disposal of Products of Biological Origin 16.4.1 Compliance with AQIS Importation Guidelines All products of biological origin entering Australia are subject to approval by the Australian Quarantine and Inspection Service (AQIS) regardless of whether the products are living or extracted and purified. For further information contact the Monash Research Office, Compliance Officer, quarantine@monash.edu. http://www.monash.edu.au/researchoffice/quarantine/ Failure to comply with these regulations will compromise the continuation of AQIS registration for this School and will be dealt with severely. AQIS REDLINE NUMBER (24 hours) 1800 803 006 16.4.2 Disposal of Quarantine Waste The autoclave in 17-249 is the only autoclave in the School suitable for autoclaving quarantine waste. All quarantine waste must be disposed of via this autoclave and run details recorded (to be audited). All users of the autoclave must be trained and have signed a Training Agreement before use. See section 20.3 for details. 16.4.3 Disposal of Biological Waste All biological wastes must be autoclaved and disposed of according to the requirements currently set by the EPA. All waste of biological origin should be treated as potentially contaminated and dealt with accordingly. The preferred disposal option is by autoclaving or incineration. If this is impractical, waste should be treated with disinfectant e.g. 5% Sodium hypochlorite. Autoclave Procedure for Biological Waste: 1. Place waste to be autoclaved into Biohazard bag. 2. Label a Thermalog strip with the next load number from the autoclaved waste record book. Place a Thermalog strip in the most heat resistant part of the autoclave waste load. E.g. Plates, tips etc - place Thermalog strip in glass jar together with biohazard bag in the autoclave bucket. 3. On completion of autoclaving, fill in the relevant sections in the Autoclave waste record book. This will include: Date Bag # 52 Load contents Thermalog result Operators signature Bag label checked Supervisors signature As required include results of counts of spore-forming bacteria. 4. Fill out adhesive labels as follows: Biological Sciences Monash University Bag # 5. Have the supervisor check the Thermalog results, adhesive label and then sign the book. Do not throw out the Thermalog until it has been checked. 6. Put autoclaved biohazard bag into black heavy duty garbage bag (i.e. double bagged) and sealed with tape printed “sterile”. 7. Stick adhesive label with details (step 5) on to the garbage bag. 8. Put into rubbish bin. 9. Stick the print out of the autoclave run in the record book and label with bag number. Currently the preferred methods of disposal within the School are: Solid Biological Waste (Non-Quarantine) All biologically contaminated waste from PC2 laboratories must be placed in labeled, baglined autoclave bins for decontamination. Currently most solid biological waste (non quarantine) is disposed of via an external contractor. Waste must be sealed inside a biological waste bag and placed into the yellow biological hazard bins. These bins must not be overfilled as the lids need to be completely shut when not being filled. There are currently bins at: Faculty Science Store, Building 53, 1 st floor and 17-212. Alternatively, this waste can be autoclaved following the guidelines for quarantine waste above. Liquid Biological Waste Liquid biohazardous waste such as bacterial cultures or other potentially contaminated solutions can be treated with 5%hypochlorite (made up freshly) for 30 minutes or autoclaved and tipped down the sink. Sharps All sharps containers are treated as biohazardous and disposed of though the Faculty of Science Store. See section 15.4 “Chemical Waste Disposal‟ for the procedure of waste disposal through the School Store. ****Note that autoclaves must be used ONLY by staffs that have been formally trained in their safe operation. See section 20.3.1 53 16.5 International Air Transport Association (IATA) Regulations There are strict regulations governing the transport of Dangerous Goods, including biologicals, by post or air freight both within Australia and overseas. Failure to follow IATA regulations will incur legal penalties. There are specific packaging guidelines for different types of goods, each package must be labeled correctly and specific documentation must be completed. Several members of the technical staff have received specific training in IATA compliance, and are able to assist on request. Contact the School Manager for details. 54 17 Working with Radiation 17.1 Useful contacts See Section 2.7 “Radiation Contacts”. 17.2 Purchase of Radioisotopes These are governed by the Health (Radiation Safety) 1983 and Regulations 1984 (SR 191) Act. All School purchases are stored and used under such licences, and we are obliged to inform OHSE of our Isotope holdings and any changes thereto. The School RSO (or in their absence the deputy RSO) must authorise all requests to purchase radioactive substances and irradiating apparatus within the school before an order can be placed. 17.3 Storage and Use of Radioisotopes 17.3.1 Radioisotope Laboratories Storage, dispensing, assaying and disposal of radio-isotopes may occur only in designated areas. If you are considering using radioisotopes please discuss your needs with the School Radiation Safety officer. All operations with radioisotopes MUST be carried out in a designated area for this type of work under the guidance of your supervisor. 17.3.2 Authorisation Before beginning work with radioisotopes, staff and students will need to consult the School’s Radiation Safety Officer. They must complete and pass training modules (2) – a practical and theory component on unsealed sources (developed by the OHSE branch). They must read and sign a declaration form indicating they will abide by the rules of the room and acquire a personal radiation monitor from the Buildings and Equipment Officer. Staff and students will not be given a monitor until the modules have been satisfactorily completed and a Risk Assessment completed. All new 55 users must be trained in experimental handling of radioactive material by the Buildings and Infrastructure Officer and that training must be documented. Signed Training sheets are to be kept. Training, General Housekeeping and Facility Rules: Buildings and Infrastructure Officer Specific procedures & protocols: Relevant lab staff 17.3.3 Radiation Safety Procedures The following general laboratory regulations apply to the handling of unsealed radioactive materials. Please note these procedures are currently under review. General laboratory rules apply, plus: On entry to the room, complete the user log sheets. The room must be checked/scanned on entry and when you leave the room. The standard of cleanliness in a radio- isotope laboratory should be much higher than normal. No music in the lab – including radios and iPods (or similar) see section 21.8. Personal protective equipment reserved for radioactive work only shall be worn at all times in the laboratory. This includes lab coats, gloves and safety glasses must be worn at all times, and must be removed before leaving the room. All radioactive preparations shall be clearly marked with details of the chemical compound, radionuclide, activity and date. The working area must be thoroughly checked for contamination after every experiment and decontaminated if necessary. When leaving the laboratory, every worker should wash their hands thoroughly. Hands, clothing and shoes shall be monitored before leaving the laboratory to ensure that no contamination is present. The following procedures must also be followed by all users of the Radiation Areas: To ensure your own safety and that of other users, it is essential that at the completion of your work you leave these rooms clean, uncontaminated and free of leftover items from experiments. If it is essential that you leave experiments running unattended, you must clean up all wastes and non-essential items before you leave, and you must also leave a notice giving details of the experiment, your name, and the time when you will return. This is your responsibility, and persistent offenders will be barred from performing this type of work. You must check your work area before you start work and when you leave using one of the Geiger-Muller counters available from the Building and Infrastructure officer. The GM tube has a delicate window, so do not drop or jolt it! Please treat the counter with respect! Do not use gas flames. All heating must be carried out using electric apparatus provided (i.e. heating blocks). Boiling reactions must NEVER be left unattended. Only o-ring tubes to be used All samples must be labeled with: Name, isotope, date and contact #‟s All items must be stored appropriately. 56 17.3.4 Personal Radiation Monitors All members of the School who work with radiation will be issued with a personal monitor (badge, or TLD), supplied by the Australian Radiation Laboratory (ARL), which records the amount of ionising radiation to which the wearer is exposed. Badges are collected every 12 weeks and returned to ARL for processing, generating a cumulative record of the wearer’s radiation exposure. Each user is given a unique number, which allow life-time exposure records to be kept. When you leave the School and/or Monash University, you should make a note of this number so that you can re-use it if you re-join the monitoring scheme at another Australian institution. You should wear your badge at all times when performing radiation work, whether or not you are actually handling radiation yourself. 17.3.5 Working outside normal working hours As far as possible, workers should try and limit their radiation work to the hours of 8am to 6pm, Monday to Friday. Should it be necessary to work outside normal working hours, all safety precautions need to be observed with extra care and the person undertaking the work MUST know what to do (i.e. how to respond, who to contact etc) in the event of an emergency. For further information, see: http://www.monash.edu.au/ohs/topics/index.html (Download “After hours work‟) 17.4 Accidents and Spills Any accident, no matter how apparently trivial, must be reported. In most cases the report should be made to The Radiation Safety Officer or in their absence, The Head of School. The School’s emergency radiation clean up kits are located in each registered lab.Please ensure you know where these are located. In any incident involving radioactive contamination, practical common sense and careful monitoring is essential. In any accident, presence of mind is desirable, absence of body is preferable! Immediate control measures in the event of a spill: 57 Minor spills of liquids should be carefully wiped up with paper towels or tissues and the bench cloth removed by working from the uncontaminated area inwards. The affected area should be monitored and further wiping or scrubbing continued until the contamination is below the Derived Working Limit (DWL). Spills must be cleaned up by the responsible person. More serious spills will probably require immediate evacuation of the area with care being taken so as not to spread the contamination. The RSO and the RPO should be notified immediately. For personal contamination, affected areas should be washed with warm water and soap. If this fails to remove contamination to below the DWL, the RPO and/or the Health Service should be notified. 17.5 Disposal of Radioactive Materials The University has strict procedures for disposing of waste radioactive substances. Each laboratory will need to arrange disposal of Radioactive Waste to the Radioactive Waste Store by contacting the Buildings and Infrastructure Officer. A key to this area is held by the RSO and the Buildings and Infrastructure Officer. Disposal guidelines for Low Level Solid Waste SOLID NON-COMBUSTIBLE RADIO-ACTIVE waste must be packed into disposal bags which have an inner plastic lining available from the Biology Store. Hypodermic syringes and needles, pasteur pipettes and other materials with sharp surfaces must be adequately packed to ensure that the waste bag is not perforated. Solvent waste and scintillation fluid in small vials (<20ml) should be placed into sealed and labeled 20-litre disposal pails, which are provided by Chemsal. Low-level aqueous waste may be disposed of to the sewer, provided that prior approval is obtained from either the RSO or RPO. Waste must be diluted with water sufficiently to ensure that the maximum concentration in the regulations are not exceeded, and drains must be clearly labeled with a radiation hazard label. All packages or containers of radioactive solid or solvent wastes should be clearly labeled with the appropriate form, identifying the type and activity of material and who is responsible for it. All full waste containers must be labeled (including date). Containers and labels are available in the room. Waste containers must only be filled to ¾. Do not continue to fill bins past this point. For further information see the OHS website: http://www.monash.edu.au/ohs/topics/radiation-users.html Documents found at the above link include: Using Ionising Radiation at Monash University Monash Radiation Safety Manual Procedures for disposal of radioactive waste 58 18 Liquid Nitrogen 18.1 Safety Liquid nitrogen is a cryogenic liquid and the hazards associated with it arise from either its very low temperature (cold burns), the nature of the gas evolved when it boils at room temperature (a powerful asphyxiant) or a combination of both (leading to fire or explosions). As liquid nitrogen vaporises it expands to 500 times its volume and can displace air. The Nitrogen gas evolved is colourless, odourless and tasteless giving no detectable warning that the atmosphere has become oxygen deficient. Therefore, liquid nitrogen work and storage areas should always be well ventilated. The hazard of cold burns can be reduced by wearing appropriate PPE. A lab coat, safety goggles and gloves should always be worn when handling liquid NO 2. Liquid NO2 must always be collected in a Liquid NO2 Dewar and the minimum amount for an experiment decanted. Most labs have a Dewar which can withstand pressure and is NOT completely sealable, to avoid explosion. If your lab does not have a liquid NO 2 Dewar, you can purchase one from a scientific supplier. Further information can be found on: OHS Information Sheet No 41 - Handling and Storage of Liquid Nitrogen http://www.monash.edu.au/ohs/topics/info-sheets/liquid-nitrogen.html 18.2 Re-filling the Dewar’s in Building 18 Room G06 If this is the first time that you have re-filled a dewar, BRING SOMEBODY WITH YOU who has filled it before and knows how to do it (If you don’t know of anyone, see the Safety Officer). Note: Take particular care navigating over uneven ground outside (going backwards over slight rises is safer than trying to push it forwards over small bumps). Wear the face mask and gloves provided when decanting the Liquid NO 2 into your small dewar. DO NOT take the lift on the way back to your lab, take the stairs. In 2013 liquid nitrogen will be available from the School of Chemistry. To arrange this you will need to contact the following persons and please provide plenty notice. Boujemma Moubaraki (safety officer) School of Chemistry ext. 54798, Or Craig Forsyth on email: craig.forsyth@monash.edu Liquid Nitrogen in Lifts – VERY IMPORTANT Putting liquid NO2 in lifts should be avoided whenever possible. Take the stairs with your small dewar. If lift transport is required for the large storage dewar; place a sign “LIQUID NITROGEN TRANSPORT IN PROGRESS. DO NOT ENTER THE ELEVATOR” around the neck of the dewar. Wheel it to the goods lift ONLY (i.e. do not use the north or south lifts in building 17). IMPORTANT....DO NOT GET IN THE LIFT WITH THE DEWAR (empty or full). Place the sign facing outwards (so anybody attempting to get in the lift can see the sign) and send to the required floor. Take the stairs to the required floor and remove the dewar from the lift 59 19 Field Trip Safety The School follows University Guidelines for all field trips. Guidelines for the health and safety during field activities in various areas (rural/urban/international) are available on the web at: http://www.monash.edu.au/ohs/topics/off-campus-activities.html And: http://www.sci.monash.edu.au/safety/field.html 19.1 Communications 19.1.1 Essential Planning Field work necessarily entails some hazards, and so in order for the School to fulfill its OH&S responsibilities towards you it is essential that we know the details of your proposed field trips, as outlined below and detailed in the policies above. This will allow us to: Alert you to external emergencies such as floods or fires; Notify you regarding personal or work-related emergencies; Raise an alarm if you fail to return at the scheduled time; and/or Take other appropriate action to ensure your safety. Before beginning any field project, you must complete a written risk assessment and ensure that it is approved by your Supervisor. The assessment should include a map showing the location of your field site(s). If necessary, a preliminary assessment can be completed, and further maps and/or procedures appended to it as the project develops. It is essential that you record the full details of each field trip, including your exact destination(s) and expected date/time of return, both on the whiteboard in your laboratory and in the folder by the car booking folder. You should also have a nominated contact person for each trip. This may be either a work colleague or a personal contact such as a partner, parent or housemate. If you will be away overnight you should arrange to phone your contact person at an agreed time each day. Your contact person should know your exact location and your expected date/time of return. They should also know what to do if you fail to return or call in at the scheduled time or if there are any incidents, including who to advise within this School. Refer to the Safety Information and Training Agreement folder in your laboratory for more details. 60 19.1.2 Communications There are a number of communication options available: The School has two mobile phones which may be booked via the online booking calendar. If you intend to rely on a personal mobile phone, please ensure that you record the number in your risk assessment, on the whiteboard in the lab, and on the record sheets next to the vehicle booking folder. 19.2 Parking of Private Cars whilst on Field Trips If you are using a school vehicle for a field trip, you may leave your car in the car parking place usually occupied by the school vehicle. You must obtain a red “Duty Vehicle” label from the General office to put on your dashboard. Without this your vehicle will be booked for illegal parking. In either case, owner onus for damage applies. 19.3 Scuba Diving & Boating Safety The University has guidelines for snorkeling/diving and boating and copies of these documents are available from the School Boating and Safety Officer. They are expected reading for any staff or student undertaking projects involving boating and/or underwater diving. Please report any faults to school diving and boating equipment, however minor, immediately to the School Boating and Safety Officer or the Deputy School Boating and Diving Safety Officer. See section 2.8 for contact details. For further information see: http://www.sci.monash.edu.au/safety/field.html The above link provides documentation for: Field activities in country and remote areas International activities Off-campus activities undertaken in urban areas For Guidelines on boat usage, a boating checklist and log see: V:\OHSE\Marine Safety 61 20 Equipment Safety, Training and Maintenance 20.1 Isolation of Unsafe Machinery or Equipment For further details, see: http://www.monash.edu.au/ohs/topics/procedures/isolation-ofequipment.pdf 62 20.2 School Facilities See the School Guide (pages 4-6) for general information on School equipment, the loaning process and how to report faults and hazards. V:/Resources/inductionManuals/School Guide 20.3 Autoclaves Autoclaves must be used ONLY by staff or students who have been formally trained in their safe operation. 20.3.1 Training The autoclaves in the School are used for steam sterilisation of liquid and solid media, equipment such as tips and tubes, and for decontamination of biological hazardous material in compliance with standards set by the EPA, AQIS, the OGTR and other regulatory bodies. The autoclaves are monitored and calibrated regularly by the designated person in charge, but all users are responsible for ensuring proper sterilisation of their own loads. Autoclave tape is used routinely to confirm exposure to a potential sterilisation temperature but it doesn't necessarily indicate that contents are sterile. Sterilisation time is the sum of the “steam penetration time” (time required for whole load to reach the set temperature), the “holding time” (minimum time required for complete sterilisation at the set temperature) and a safety margin to allow for packaging, loading or operational faults that may not have been detected. Common reasons for the failure of sterilisation are: • Insufficient sterilising time or temperature. • Inappropriate loading of the chamber causing air to be trapped. Articles except those containing aqueous solutions should be positioned so that air can escape by downward displacement. • Inappropriate packaging of articles to be sterilised. Packaging material must be permeable to entry of steam and removal of air and must be contamination-proof during subsequent storage. Volumes of liquid should be kept as small as possible. Please remember that bottles should be filled only three-quarters full. Commonly used sterilisation times are: • Media, glassware, etc. - 20 mins at 121°C/15psi (101.3 kPa). • Contaminated soil - 60 mins at 121°/15psi (101.3 kPa). Autoclaving Biological Waste Thermalog strips must be included in every waste load that goes through the autoclaves to ensure that you are obtaining sterilisation. A waste load refers to anything that is to be thrown in an ordinary rubbish bin or tipped down the sink after being autoclaved. Thermalog strips use a chemical reaction to indicate “safe” and “unsafe” reactions. “Safe” indicates sterilisation has occurred. “Unsafe” means that sterilisation wasn't attained. 63 Strips should be placed into the middle of loads to ensure that the entire load reaches sterilisation. Water It is important to ensure that the Autoclave has adequate water before starting a run. Tap water and distilled water can be used, but not MilliQ (as it is ion free). Specifications for refilling with water vary on different autoclaves. See the manufacturers‟ manual or ask a trained person if you are unsure. Safety To safely open the door of an autoclave, the pressure must be at zero (kPa) and the temperature below 60˚C. Bottles must never be filled more than ¾ full and lids should be left on loosely to allow steam to escape and prevent bottles exploding. The department has several autoclaves varying in size and sophistication. Therefore, although you have completed the basic training and signed the Training Agreement, it is very important to seek advice from a trained person before using a different autoclave for the first time. Person appointed responsible for Training and Records Each autoclave has a responsible person appointed and their name clearly visible on the autoclave. This person is responsible for: Training users – this should include: Familiarity with the machine, its controls and the requirements of the manufacturers handbook Operating rules and limitations Emergency shutdown procedures and incident reporting Record keeping, log books Other requirements related to chemical or biological safety Keeping records of authorised users: Name, date trained and authorised Ensuring records are maintained Inspecting the autoclave for wear and tear Maintenance, contacting the Buildings and Infrastructure Officer to arrange repairs For information and guidelines on autoclaving waste, see: For further information, instruction manuals for each autoclave can be found in the training agreement folder (within close vicinity to the machine) or you can contact the Buildings and Infrastructure Officer. Trainees and the appointed Trainer must co-sign a Training Agreement in the appropriate section of the laboratory folder before the Trainee using an Autoclave. 64 20.3.2 Hazards, Faults and Maintenance All autoclaves are on a maintenance agreement and serviced in January and July each year. The person appointed responsible for the autoclave performs regular checks for general wear and tear. It is the responsibility of the user to report any hazards or faults observed during routine use. Autoclaves are pressure vessels and are potentially very dangerous. Faults or potential hazards must be reported immediately. To report a fault or hazard, contact the person appointed responsible for that autoclave. 20.4 Centrifuges The School has many centrifuges, varying in size, age and sophistication. These machines are potentially dangerous and all first-time users MUST be trained by the person-incharge. For training on high speed centrifuges see the Buildings and Infrastructure Officer or for training on bench top centrifuges see the laboratory’s staff. Bench Top Centrifuges Must be securely fixed to the bench top before use Visually inspect the rotor prior to use Hair nets to be used if hair is past chin level Clean away any moisture on the rotor on each occasion the machine is used Any spill in a centrifuge rotor must be immediately mopped up and decontaminated with 70% Ethanol (NOT Sodium Hypochlorite). Major spills must be reported so that the centrifuge can be inspected for potential damage to the drive assembly. Trainees and the appointed Trainer must co-sign a Training Agreement in the appropriate section of the laboratory folder before the Trainee uses the Centrifuge. Person appointed responsible for Training and Records is responsible for: Training users – this should include: Familiarity with the machine, its controls and the requirements of the manufacturers handbook Operating rules and limitations, rotor maximum speed Inspect the rotor prior to use Load balancing placement and security Emergency shutdown procedures and incident reporting Record keeping, log books Other requirements related to chemical or biological safety 65 Keeping records of authorised users: Name, date trained and authorised Ensuring records are maintained Inspecting rotors, interlocks of lids and interlocks to speed control and recording Maintenance (arrange servicing through the workshop every 2 years), contacting the Buildings and Equipment Officer to arrange repairs Marking the maximum allowable rotor speed on the speed controller 20.5 Fume Hood and Laminar Flow 20.5.1 Fume Hood Training A fume cupboard is essentially a ventilated box with an adjustable work opening. It provides extraction to remove any fumes produced within the box. It is designed to have laminar flow through the front opening, i.e. the flow is to be even and non-turbulent through the open face of the cupboard Whenever anything is placed within the fume cupboard, it introduces turbulence into the cupboard. This means that the containment of fumes may be affected. If a fume cupboard is not used in the proper manner then there may be situations in which fumes escape out of the front of the fume cupboard towards the user instead of being drawn away from the user, especially with heavier vapours such as formaldehyde. ALL TRAINEES MUST PRINT THE DOCUMENT BELOW PRIOR TO THE TRAINING SESSION: http://www.monash.edu.au/ohs/topics/info-sheets/fume-cupboards.html Guidelines for working in fume cupboards The following details need to be considered to ensure that the fume cupboard's performance is not compromised: Do not work within 10 centimetres of the leading edge. The larger the item, the further back it needs to be within the fume cupboard to overcome the turbulence created Do not place storage items behind the area you are working in. This is of particular importance where a Perspex screen or lead bricks are used for radioisotope work Minimise the number of items stored within the fume cupboard. If hazardous materials must be stored, secondary containment (e.g., a spill tray) must be used to ensure compliance with trade waste agreements in case of accidental spillage Do not put large equipment, such as ovens, in the fume cupboard, as they block the baffles and produce regions of zero or low flow in the workspace 66 Always have the sash at the recommended height when the cupboard is in use. This is marked on a yellow sticker at the side of cupboard Minimise traffic past the front of the fume cupboard as this can cause turbulence, which may result in fume escape Do not use fume cupboards with a porous bench surface (e.g. terracotta tiles) for work with radioactive material Do not open windows, which may create draughts in the vicinity of the fume cupboard If doors are within 1 metre of fume cupboards they should be kept closed during use of the fume cupboard The make-up air supply and room ventilation should be switched on whenever the fume cupboard is in use Trainees and the appointed Trainer must co-sign a Training Agreement in the appropriate section of the laboratory folder before the Trainee uses the Laminar Flow cabinet. 20.5.2 Laminar Flow Training A laminar flow cabinet is an enclosed bench which blows sterile air in a laminar flow towards the user to prevent contamination of a sample. As the air leaving the cabinet flows directly out of the cabinet without filtration, it is essential that the work performed in the cabinet does not present a hazard to the user, other laboratory members or the environment. Ethanol is commonly used to clean the bench and a flame in the cabinet as part of a sterile technique. DO NOT KEEP THE ETHANOL IN THE CABINET (FIRE HAZARD). The cabinet is fitted with a UV light to sterilise the contents when not in use. This should be switched off before removing the cabinet cover. Any questions unable to be answered by your supervisor or appointed trainer, please contact the Buildings and Infrastructure Officer. Trainees and the appointed Trainer must co-sign a Training Agreement in the appropriate section of the laboratory folder before the Trainee uses the Laminar Flow cabinet. 20.6 Incubators and Ovens There are a number of incubators in the School, some of which are connected to CO2 cylinders for controlled-atmosphere tissue culture work. Some manuals describing their care are kept in the Manuals Library, located in the Utilities Room. When starting an incubator all the switches should never be put on simultaneously; ensure you follow the correct sequence as detailed in the manual. All newly purchased incubators/ovens MUST be fitted with over temperature cut-off switches. See the Safety Officer for further information. Malfunctions can be reported to the Buildings and Infrastructure Officer. 67 20.7 Constant Temperature (CT) Cabinets and Rooms For information on alarm systems, power supply issues, changing the settings and use of freezer rooms and cold rooms, see the School Guide pages 8-9. It is not necessary to complete a Training Agreement to use these facilities, however it is important that you read the School Guide to understand safety procedure and comply with facility etiquette. 20.8 Fridges and Freezers Intrinsically safe refrigerators/freezers have no sources of ignition within the cabinet, i.e. all thermostats, light and door switches, defrosting heaters, recirculating fans and motors are located in an isolated position outside the refrigerator/freezer cabinet. These refrigerators/freezers are safe for the storage of flammable liquids. All refrigerators/freezers must be labeled with the approved labels, denoting whether they are intrinsically safe or not intrinsically safe. These labels are available from the Safety Officer. - Chest freezers are intrinsically safe - Cold rooms must not be labeled as intrinsically safe See the Safety Officer if you are unsure of the classification of your fridge or freezer. 20.9 Balances – Calibration and Cleaning Balances should be kept clean for optimum performance. A paint brush can be used to brush away spills of dry chemicals which can then be cleaned from the bench in accordance to the specific chemicals’ clean up procedure (see MSDS if Hazardous). There is a set of calibration weights kept in the office of the Buildings and Infrastructure Officer. Calibration instructions are specific to the make and model of your balance and should be found in the manufacturer’s instruction manual. 68 20.10 Pipettes 20.10.1 Ergonomic Use Manual Plunger-Operated Pipettes For advice on selecting the right pipette for you and using it in an ergonomic way, see section 14.3 “Ergonomics in your work space”. Glass Pipettes Glass pipettes a source of common lab injuries. For information on safe use, alternative equipment and training, see the Monash University Hazard Alert: http://www.monash.edu.au/ohs/topics/hazard-alerts/glass-pipettes.html 20.10.2 Calibration and Cleaning of Manual Plunger-Operated Pipettes It is always best to read the manufacturer’s instructions before attempting to clean or calibrate pipettes. Most companies offer a cleaning and calibration service to maintain original accuracy and performance (see appropriate website). As a general guide: Cleaning (For common brands such as Gilson or similar). Wear gloves, lab coat and goggles. Dismantle the pipette in a tray by removing the tip ejector and unscrewing the shaft. As the shaft comes off take careful notice of the positioning of all parts to assist with re-assembling later. Be careful not to lose the small parts which become free (spring, o-ring, ejector button). To clean, soak all parts except the main body of the pipette in 96% ethanol. There are small bushes to use in the calibration/cleaning kit. Dry all parts. Examine the piston and o-ring before re-assembly. If there are any marks, blemishes or corrosion on the piston, the pipette is less likely to be accurate and reliable. A damaged piston is not usually replaceable and rather the whole pipette is replaced if accuracy is low. If the o-ring looks worn (even slightly) replace it. If it looks like new, do not replace it (for cost reasons). Re-assemble the pipette. Calibration The kit containing the calibration tool (for Gilson) and instructions on how to calibrate are in the Buildings and Infrastructure Officer’s office. 69 20.11 Specialised Laboratory Equipment Any piece of equipment that is specific to your laboratory and requires training of new users must have an Equipment Training Agreement. New sheets can be found in the “Training Agreement‟ section of Safety Folder 1 (Safety, Training and General Information) or V drive: V:\OHSE\Labels, Signs & Templates\Templates\Equipment Training Agreement Requirements of the Trainee in section 20.12.2 (below) must be met before the Training Agreement is signed and equipment is used. If you have a new piece of equipment and are unsure of whether users need to sign a Training Agreement, contact the Safety Officer. 20.12 Equipment Responsibilities and Training 20.12.1 Equipment Responsibilities Each piece of equipment must have a responsible person appointed and their name in the designated space of the first page of Safety Folder 1 (Safety, Training and General Information). This appointed person is responsible for: Training users – see below. Keeping records of authorised users (Training Agreements) Ensuring records are maintained Maintenance 20.12.2 Equipment Training Trainees and the appointed Trainer must co-sign a Training Agreement in the appropriate section of Laboratory Safety Folder 2 before the Trainee uses the Laboratory Equipment. As a part of the training for common laboratory equipment such as autoclaves, centrifuges, fume hoods and laminar flows the trainee MUST read the appropriate section of this manual. If you have training instructions for any piece of equipment which you think should be included in this Safety Manual, please email the School Manager with the suggestion. After the explanation of safety and user guidelines by the trainer, the trainee must display with competence: Familiarity with the machine, its controls and the requirements of the manufacturers handbook Operating rules and limitations Emergency shutdown procedures and incident reporting Record keeping, log books 70 Other requirements related to chemical or biological safety Day-to-day maintenance The trainee must sign an Equipment Training Agreement in Safety Folder 2 BEFORE using the equipment. 71 21 Policies, Procedures & Guidelines 21.1 Emergency Response Procedures - Business Hours 72 21.2 Emergency Response Procedures – After Hours 73 21.3 Fire Extinguishers Source: http://a.cdn.fpaa.com.au/information/docs/Portable%20Fire%20Extinguisher%20Guide.pdf 74 21.4 First Aid Treatment Speed is important Remember DRSABCD: • Danger (to you, the injured person or anyone else?) • Response (is the injured person conscious?) • Send for help (Call 000 for an ambulance) • Airway (is it clear?) • Breathing (is the injured person breathing?) • CPR (If not breathing, start CPR) • Defibrillation (apply defibrillator) Protect from further damage Immediately remove contaminated clothing Flush the affected area with plenty of water Do not give first aid, expired air resuscitation (EAR) or cardiopulmonary resuscitation (CPR) unless you have been appropriately trained to do so Eyes – First Priority! Wash continuously for 20 minutes with eye stream or water Flush well under the eye lids Do not use PEG (Polyethylene Glycol) in eyes! Seek Urgent Medical Attention Skin Inhalation After flushing the affected area with plenty of water apply PEG 300 or 400 (polyethylene glycol), massaging well into skin using lint cloth. Change the PEG solution frequently by thoroughly washing the lint cloth or replacing it. Alternate PEG with methylated spirits (Health Service or Ambulance only) Continue treatment until the skin returns to its original colour. If the skin is broken, cover with a dressing. Seek urgent medical advice if a large area of skin is exposed, skin colour does not return or treatment has been delayed. Continue to use PEG until medical attention is obtained. Fresh Air Oxygen treatment and send to hospital if breathing is difficult Swallowed Give a Glass of Water and send to hospital urgently. NB: A Phenol First Aid Kit containing the MSDS and a first aider should accompany all injured people to the hospital or doctor. 75 21.5 Screen-Based Equipment Exercises One of the more common complaints which staff experience and is reported to the Safety Officer for investigation are problems associated with spending long periods at a computer, microscope, laminar flow or other static task. If you are to avoid aching muscles and tired eyes, it is important that you adopt certain work practices. The following may help: Check your posture Adjust the chair height so that your arms are approximately parallel with the floor If the front of the chair is causing pressure on the back of your thighs or behind your knees, you may need a footstool. Adjust the chair backrest to support the lower back while you sit in the typing posture Locate the computer screen approximately one full arm’s length away and position it so that your line of sight to the screen is slightly below horizontal Relax the shoulder muscle and check your wrist and forearms are still approximately parallel with the floor, then commence typing. Relax those muscles! As muscles tire from holding the keying posture they need to relax regularly during the day. 2-3 minute breaks are recommended every 15-20 minutes When muscles feel tight or tired, it is good to stretch, relax, stretch, and relax the muscle area to relieve the tiredness Exercise can be done regularly and unobtrusively when required. A total of 4 hours (not including breaks) of intensive keyboard work is the maximum time recommended each day. Are your eyes tired? General room lighting is usually suitable for working at a computer. The computer should be located so that you do not face directly at a window when looking at the screen, nor should a window be directly behind you. If overhead lights are reflected in the screen, tilt the screen forwards to minimise the reflections. The eyes need to relax from working at a fixed focal length hence look away from the computer screen regularly. 76 21.5.1 Screen-Based Equipment Exercises http://www.monash.edu.au/ohs/topics/index.html (Download Computer use and Ergonomics). Ergonomics - Computer user guidelines 77 21.6 Insurance 21.6.1 Student Personal Accident Insurance Policy All enrolled students at Monash University are automatically members of a personal accident insurance scheme, which is funded through the Amenities Fee paid by all students. The current policy is restricted to claims resulting from accidents while a student is engaged in university/course-related activities. The policy is designed to assist in minimising the financial burden associated with “physical or bodily injury which happens as a result of external violence”. Costs associated with illness or disease are not covered under the terms of the policy. Benefits include: Reimbursement of non-Medicare expenses to a maximum of $2,000 Overseas medical expenses to a maximum of $50,000 Weekly injury benefits for income earners Domestic help and/or childcare for non-income earners Home tutorial benefits for full-time students Bed care benefits An excess of $150 per claim applies, together with a number of other conditions. For more details, contact the Sports and Recreation Office on 9905-4118 or see http://www.monash.edu.au/ohs/topics/insurance-support.html Other Forms of Insurance Public liability and general indemnity insurance is provided to all Monash students whilst they are engaged in activities relating to their studies at Monash. This includes Honours and postgraduate students engaged in laboratory work and off-site field work, and provides the same level of cover as is given to staff. Travel insurance for postgraduate students on authorised business travel (e.g. conference attendances or overseas field work) is provided under the University’s Travel insurance policy. For more details, see: http://sci.monash.edu/postgrad/travel1.html Private travel insurance, including hospital and medical insurance, is available from the STA Travel offices on the Caulfield and Clayton campuses. 78 21.7 Use of headphones, earphones, ear buds in the Workplace Monash University has a duty of care for the health and safety of staff, students, contractors and visitors. It recognises that many staff and students use audio equipment (audio-visual & multi-media production etc.) and other devices (MP3 players, iPods etc.) with headphones/earphones/earbuds and that this may be directly associated with work or study. This information sheet aims to provide advice to ensure that the use of these devices does not impact on the health and safety of users and others at Monash University. The following points should be considered by all University staff and students to minimise the potential health and safety impacts associated with the use of audio headphones/earphones/earbuds. General • If the wearer’s situational awareness is reduced to the extent that their health and safety is compromised by the use of these devices e.g. they cannot hear emergency alarms, calls for help etc., these devices must not be used. • The use of high volumes can cause permanent noise-induced hearing loss. If someone standing nearby can hear what the wearer is listening to, the volume is too loud. • Staff and students should consider using a single ear piece whenever possible, to assist in maintaining awareness of what is happening around them. • Headphones/earphones/earbuds should be maintained in a clean and hygienic state. It is important to follow the manufacturer’s instructions when cleaning these devices due to the potential for damage to the electrical/electronic components contained in the unit. • Internal units such as earbuds should be single user only due to hygiene issues. Activities requiring the use of headphones/earphones/earbuds • Where activities require the wearing of headphones/earphones/earbuds, a risk assessment must be completed prior to their use. This is especially important in laboratories, workshops, studios or any other area where harmful biological, chemical or other substances and materials e.g. infectious or toxic substances, metal particles etc are used. • If a risk assessment indicates there is no alternative but to use the headphones/earphones/earbuds, approval must be obtained from area managers, supervisors, etc, prior to the use of any such items in the workplace. • Headphones/earphones/earbuds must be stored in such a way that minimises the risk of them being contaminated by biological, chemical or other substances and materials. • Ideally external units such as headphones and earphones should be single user. Where this is not practical it is important to insure that the units are cleaned according to manufacturer’s instructions prior to transfer between individuals. • Headphones/earphones/earbuds must not be repeatedly touched, adjusted or re-fitted to the ear where there is risk of contamination from biological, chemical or other substances and materials For further information see: http://www.monash.edu.au/ohs/topics/info-sheets/headphones.html Or contact your local OHS&E Consultant or Occupational Health and Safety by phone on 990 51016 or by email on ohsehelpline@adm.monash.edu.au. 79 21.8 Other OHSE Policies and Documents For a complete list of OHS&E policies, and other related documentation, see http://www.monash.edu.au/ohs/topics/index.html A number of useful links are also available the following web sites: School of Biological Sciences: V:\OHSE Faculty of Science http://www.sci.monash.edu.au Occupational Health, Safety and Environment branch http://www.monash.edu.au/ohs/ “Ask Monash” https://monash.custhelp.com/ 80