Food Chemistry 121 (2010) 191–195
Contents lists available at ScienceDirect
Food Chemistry
journal homepage: www.elsevier.com/locate/foodchem
Changes of hormone-sensitive lipase (HSL), adipose tissue triglyceride lipase
(ATGL) and free fatty acids in subcutaneous adipose tissues throughout the
ripening process of dry-cured ham
Shan Xiao a, Wangang Zhang b, Yong Yang a,c, Changwei Ma a,*, Dong U. Ahn b,d, Xiang Li e,
Jiankun Lei e, Min Du f
a
College of Food Science and Nutritional Engineering, China Agricultural University, Beijing 100083, China
Department of Animal Science, Iowa State University, Ames, IA 50011-3150, USA
c
Department of Food Science, Sichuan Agricultural University, Ya’an 625014, China
d
Major in Biomodulation, WCU, Seoul National University, Seoul 151-921, Republic of Korea
e
Dongheng Group, Fuyuan County, Yunnan Province 655500, China
f
Department of Animal Science, University of Wyoming, Laramie, WY 82070, USA
b
a r t i c l e
i n f o
Article history:
Received 16 September 2009
Received in revised form 20 October 2009
Accepted 9 December 2009
Keywords:
Dry-cured ham
Adipose triglyceride lipase (ATGL)
Hormone-sensitive lipase (HSL)
Adipose tissue
Free fatty acids
a b s t r a c t
Changes in adipose triglyceride lipase (ATGL) and hormone-sensitive lipases (HSL) in subcutaneous adipose tissue of Xuanwei ham at different ripening stages were studied. Green hams were salted for 40 d
and then ripened in a ventilated chamber for 15 months.
Adipose triglyceride lipase and HSL could be detected during the whole ripening period of Xuanwei
ham, but their protein levels decreased as the ripening time increased. The decrease of lipase proteins
could be attributed to the decrease in protein synthesis as ripening time progressed. The level of free fatty
acids (FFAs) increased at the first stage but HSL protein remained constant indicating that HSL was more
important than ATGL for adipose tissues lipolysis. The main FFAs produced during the ripening period of
Xuanwei ham were oleic acid, linoleic acid and palmitic acid, suggesting that the triacylglycerols containing these three FFAs were preferential substrates for ATGL and HSL.
Ó 2010 Elsevier Ltd. All rights reserved.
1. Introduction
Adipose tissues in dry-cured hams play important roles in flavour development and significantly contribute to the nutritional
value of ham (Wood et al., 2007). In addition, the colour, firmness
and thickness of adipose tissues can affect consumer’s acceptance
of dry-cured ham (Coutron-Gambotti & Gandemer, 1999).
During the long process under mild ripening conditions of drycured ham, lipids undergo intense lipolysis that involves a set of
endogenous enzymes followed by auto-oxidation, which contributes to the formation of aromatic volatile compounds (Marra, Salgado, Prieto, & Carballo, 1999; Toldrá, 1998; Toldrá, Flores, & Sanz,
1997). Thus, endogenous lipolytic enzymes are very important for
flavour development and final flavour quality of ham (Toldrá & Flores, 1998). Two main lipase systems are involved in the lipolysis of
subcutaneous adipose tissues: neutral and basic lipases described
* Corresponding author. Address: Laboratory of Meat Science, College of Food
Science and Nutritional Engineering, China Agricultural University, No. 17, Qinghua
East Road, Haidian, Beijing 100083, China. Tel.: +86 10 62737643; fax: +86 10
62731265.
E-mail address: chwma@cau.edu.cn (C. Ma).
0308-8146/$ - see front matter Ó 2010 Elsevier Ltd. All rights reserved.
doi:10.1016/j.foodchem.2009.12.029
as hormone-sensitive lipases (HSL) and lipoprotein lipases (LPL),
respectively (Belfrage, Frederikson, Strälfors, & Tornqvist, 1984;
Martín, Ruiz, Flores, & Toldrá, 2006; Motilva, Toldrá, Aristoy, & Flores, 1993). By controlling these enzymes, therefore, we may be able
to standardise processing procedure and improve the quality of
dry-cured ham (Toldrá, 2006). However, only few studies have
been conducted on the enzymes responsible for the lipolysis in
dry-cured ham (Coutron-Gambotti & Gandemer, 1999; Vestergaard, Schivazappa, & Virgili, 2000; Zhou & Zhao, 2007).
In recent years, a novel lipase named ATGL (adipose triglyceride
lipase, also called desnutrin), which plays a key role in triacylglycerol (TAG) breakdown (lipolysis) in adipocytes, was discovered
(Jenkins et al., 2004; Villena, Roy, Sarkadi-Nagy, Kim, & Sul,
2004; Zimmermann et al., 2004). This new and exciting discovery
challenges the concept that has been widely accepted for more
than 30 years: HSL is the rate-limiting enzyme responsible for triacylglycerol breakdown. This also suggests that both ATGL and HSL
are the major lipases involved in the lipolysis of dry-cured ham
during processing.
China has a long history of producing dry-cured hams. Xuanwei
ham in Yunnan province together with Jinhua ham in Zhejiang
province and Rugao ham in Jiangsu province are the three famous
192
S. Xiao et al. / Food Chemistry 121 (2010) 191–195
hams in China. Yunnan province is located in the southwest frontier of China at 2000 m above sea level and enjoys pleasant
spring-like weather for most of the year (average annual temperature 14 °C). Due to the unique climate, geography and traditional
processing techniques, Xuanwei ham has developed its unique flavour traits.
The objective of this study was to analyse adipose triglyceride
lipase profiles during Xuanwei ham maturation in order to understand the lipolysis of dry-cured hams better. In this study, ATGL
and HSL protein expressions and FFA values were measured in subcutaneous adipose tissues during the 15-months ripening period of
Xuanwei ham. The changes of pH, aw and NaCl concentration during ripening period were also analysed to explore their effects on
lipases.
2. Materials and methods
2.1. Processing of ham and sampling
Dry-cured hams were produced by Dongheng Group (Yunnan,
China). Wujin X Yorkshire cross-bred pigs were slaughtered at
12–14 months of age with body weight between 90–100 kg. The
green hams were held at 1–4 °C and 50–60% relative humidity
(RH) for 10–12 h. Then, the hams were salted three times, 2.5%,
3.5%, and 2% (w/w), respectively, and the total duration of salting
was 40 d at 1–7 °C and 70–80% RH. After soaking in cold water
for 6–8 h and washing the excess salt on the surface of the hams,
they were hung on strings in a ventilated chamber (temperature
ranging from 19 to 24 °C and 58–80% RH) and ripened for
15 months. The external subcutaneous adipose tissues (about
1.5 cm) covering the Biceps femoris muscle were removed from
six hams at 0, 3, 6, 9 and 15 months of ripening period, vacuumpackaged in oxygen impermeable bags (O2 permeability,
9.3 mL O2/m2/24 h at 0 °C) and stored at 80 °C until analysed.
Water activity (aw) was measured using a water activity metre
(Testo, IKA T10, Germany). pH was measured directly in the subcutaneous fat with a waterproof pH-metre (HI 9025, HANNA, Italy).
NaCl concentration was determined using a standard AOAC method (1999).
2.2. Western blotting analysis
About 1 g tissue was pulverised in liquid nitrogen and homogenised with 5 ml of ice-cold buffer containing 50 mM Tris–HCl
[pH 7.4]; 150 mM NaCl; 1% NP-40; 0.25% sodium deoxycholate;
1 mM EDTA; 1 mM PMSF; 2 lg ml 1 leupeptin; 2 lg ml 1 pepstatin A; 2 lg ml 1 aprotinin; 500 lg ml 1 benzamidine; 1 mM
Na3VO4; and 1 mM NaF. The homogenate was vortex mixed for
60 s and put on ice for 45 min. They were then homogenised twice
using a polytron for 15 s each, and then centrifuged at 14,000g for
10 min at 4 °C. The supernatant was transferred to a fresh tube and
total protein content of the supernatants was determined using the
Bradford method. An aliquot of supernatant was transferred to a
test tube, an equal volume of reducing buffer (0.1 M Tris–HCl
[pH 6.8]; 2% SDS; 20% glycerol; 10% b-mercaptoethanol; 0.1% bromophenol blue) added, and boiled for 5 min. Proteins were separated using 12% SDS–PAGE gel and then transferred to a
nitrocellulose membrane. The membrane was later cut into two
pieces according to the molecular weight of ATGL and HSL. They
were then incubated in a block solution containing 5% nonfat dry
milk in PBST (80 mM NaH2PO4; 20 mM NaHPO4; 100 mM NaCl;
0.1% Tween-20) for 1 h, followed by incubating (1:1000, diluted
with PBST containing 5% nonfat dry milk) with rabbit anti-ATGL
antibody (Cell Signalling, #2138, USA), and rabbit anti-HSL antibody (Cell Signalling, #4107S, USA) separately, with gentle agita-
tion at 4 °C overnight. Then, membranes were incubated for 1 h
with the HRP-conjugated secondary antibody (Cell Signalling,
#7074, USA) and then washed thoroughly with PBST. Antigen–
antibody complexes were visualised using enhanced chemiluminescence ECL (GE healthcare, HP79NA, UK) and exposed using a
16-bit charge-coupled device camera (FluorChem8800; Aopha
Innotech Corporation, San Leandro, CA). The density of bands on
Western blotting was measured using a densitometer (BandScan,
Glyko, Novato, CA).
2.3. Lipid extraction and FFAs determination
Total lipids were extracted from 5 g of adipose tissues with
chloroform/methanol (2:1; v/v) according to the method of Folch,
Lees, and Stanley (1957). Then the free fatty acids were purified
from the total lipids using a strong basic anion exchange resin
(Amberlite A26) which described by Gandemer, Morvan-Mahi,
Meynier, and Lepercq (1991). Lipids (50 mg) were mixed with
15 ml acetone/methanol (2:1; v/v), to which heptadecanoic acid
(C17:0) was added as an internal standard. The mixture was shaken (120 rpm) with 150 mg of strong basic anion exchanger resin
(Amberlite A26, Sigma, St. Louis, USA) for 30 min and then the solvent was removed. The free fatty acids in the resin was eluted with
acetone/methanol (2:1; v/v) 5 times and dried under N2 stream.
The FFAs were methylated with 2 ml of Boron Trifluoride-methanol and then the FFA methyl esters were analysed using a gas chromatography (HP 6890, Wilmington, DE, USA). A capillary column
(HP-Innowax, 30 m long, 0.32 mm internal diameter, 0.25 lm film
thickness, Agilent Technol., Wilmington, DE, USA) was used. The
oven temperature was programmed at 200 °C for 2 min, increased
to 202 °C at 0.4 °C min 1, increased to 207 °C at 0.7 °C min 1, and
then held for 2 min. N2 (flow rate 2.81 ml min 1) was used as a carrier gas. The compounds were detected using a flame ionisation
detector at 275 °C. Fatty acid standards (Sigma, St. Louis, USA)
were used to identify the FFAs and results were expressed as mg
FFA per gram extracted lipids.
2.4. Statistical analyses
The changes in enzyme levels, chemical parameters (pH, aw and
NaCl concentration) and FFAs characteristics were analysed using
SAS program (Version 8.1, SAS Institute Inc., Cary, NC). The analysis
of variance (ANOVA) and the student t-test were used to identify
significant differences.
3. Results and discussion
3.1. Adipose tissue HSL and ATGL protein levels as affected by
processing factors
Our study, for the first time, examined the amounts and
changes of adipose tissues HSL and ATGL protein expression levels
during ageing of Xuanwei ham. According to recent reports, HSL,
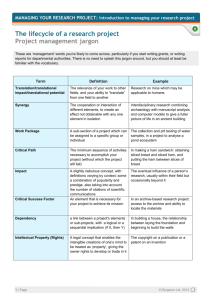
largely found in cytosol, hydrolyses diacylglycerol (DAG) to generate monoacylglycerol (MAG) (Granneman et al., 2006). In the current study, HSL was detected throughout the 15 months of
ripening period (Fig. 1A). This result agrees with Coutron-Gambotti
and Gandemer (1999) and Motilva et al. (1993) who reported that
neutral lipase, described as HSL, remained active over 12 months
processing period. During the first 3 months of ripening period,
HSL protein level decreased slowly, and then decreased rapidly
(41.94%, p < 0.01) between 3 and 6 months. From 9 to 15 months,
the HSL level sharply decreased (88.64%, p < 0.01, Fig. 1A). ATGL,
a newly identified lipase found in cytosol and lipid droplets, catalyses the hydrolysis of the first ester bond in TAG (Granneman et al.,
193
S. Xiao et al. / Food Chemistry 121 (2010) 191–195
0
(A)
3
9
6
15
HSL
1.2
3
6
9
15
ATGL
a
a
1.2
0.8
ATGL relative protein
expression level
1.0
HSL relative protein
expression level
0
(B)
b
b
0.6
0.4
c
0.2
a
1.0
b
0.8
c
0.6
c
0.4
d
0.2
0.0
0.0
0
3
6
9
ripening time (months)
15
0
3
6
9
ripening time (months)
15
Fig. 1. Western blotting analysis of HSL and ATGL protein contents in adipose tissue during Xuanwei ham maturation. (A) HSL relative protein level in adipose tissue during
Xuanwei ham maturation. (B) ATGL relative protein level in adipose tissue during Xuanwei ham maturation. Means with different letters differ significantly (p < 0.01), n = 6.
ATGL and HSL protein levels during ripening. During the first
stage of process (0–3 months) when ATGL and HSL had the maximum protein levels, the amount of FFAs increased 29.51% (from
60.55 to 78.42 mg g 1, p < 0.01, Table 1 and Fig. 1). The levels of
FFAs in the following stages were 17.5% (3–6 months), 8.31%
(6–9 months) and 10.29% (9–15 months), respectively. The rate of
FFAs increase at the later stages (6–15 months) was lower than
that of the earlier stages, which were attributed partly to the
lower lipase levels during the later stages. In this study, even at
the last ripening phase (9–15 months), we found increased accumulation of FFAs, which were consistent with our findings that
the two lipases were detected and most possibly had activities at
that time.
It is undisputed that lipolysis is one of the most important reactions for aroma volatiles production and FFAs are known to be
associated with flavour in aged meat (Motilva & Toldrá, 1993).
Therefore, it is reasonable to speculate that both ATGL and HSL
play important roles for the flavour development in dry-cured
2006). The results of our study indicated that ATGL could be
detected up to 15 months of ripening period (Fig. 1B). The rapid
decrease (42%, p < 0.01) in ATGL was observed during the first ripening phase (0–3 months), and significantly decreased (29.31%)
during the second ripening phase (3–6 months). It decreased at a
slower rate during the third ripening phase (6–9 months) while a
rapid decrease (60.66%, p < 0.01) was observed at the final phase
(9–15 months). In vitro studies showed that ATGL together with
HSL was quantitatively the most important lipases found in adipose tissues (Shen, Patel, Yu, Jue, & Kraemer, 2007). They were
responsible for more than 95% of the TAG hydrolysis activity while
other lipases (known or unknown) play a minor role in fat cell
lipolysis (Motilva et al., 1993; Schweiger et al., 2006; Vestergaard
et al., 2000). Therefore, it is suggested that these two lipases are
mainly responsible for the FFA accumulation during ham processing. Table 1 showed that the amount of FFAs increased significantly
during the whole sampling period from 60.55 to 110.07 mg g 1
(p < 0.05), and the kinetics of FFAs release were consistent with
Table 1
Free fatty acids changes in adipose tissue during Xuanwei ham maturation.A
Ripening time (months)
Fatty acids
0
3
6
9
15
Sig.B
mg/g lipids
A
B
Free fatty acids
C12:0
C14:0
C16:0
C18:0
C20:0
Total saturated
0.78 ± 0.50
1.07 ± 0.39b
10.67 ± 3.20b
4.15 ± 1.60b
0.85 ± 0.39
17.53 ± 5.63b
0.79 ± 0.49
1.90 ± 0.12ab
16.22 ± 1.65ab
4.95 ± 0.75ab
1.41 ± 0.21
25.27 ± 1.72ab
0.69 ± 0.15
2.79 ± 0.67a
21.97 ± 4.35ab
5.49 ± 0.94ab
1.10 ± 0.18
32.03 ± 6.22ab
0.54 ± 0.17
2.19 ± 0.51ab
21.02 ± 2.88ab
5.16 ± 0.65ab
1.27 ± 0.50
30.18 ± 3.70ab
0.66 ± 0.07
2.41 ± 0.69a
26.66 ± 6.62a
7.18 ± 0.81a
1.53 ± 0.16
38.45 ± 8.26a
C16:1
C18:1
C20:1
Total monounsaturated
2.04 ± 0.55
28.37 ± 7.23c
1.28 ± 0.55
31.69 ± 8.18b
2.57 ± 0.14
33.81 ± 5.95bc
1.27 ± 0.17
37.65 ± 6.12ab
4.08 ± 0.74
40.75 ± 5.32abc
1.20 ± 0.08
46.04 ± 5.95ab
3.64 ± 1.13
45.54 ± 2.37ab
0.91 ± 0.35
50.09 ± 3.10a
3.75 ± 0.94
48.98 ± 7.74a
0.79 ± 0.12
53.51 ± 8.79a
C18:2
C18:3
C20:4
Total polyunsaturated
9.47 ± 2.11
0.88 ± 0.26b
0.97 ± 0.60
11.33 ± 2.95
12.19 ± 1.18
2.24 ± 0.53ab
1.07 ± 0.68
15.49 ± 0.71
11.24 ± 1.78
2.02 ± 1.03ab
0.81 ± 0.22
14.07 ± 0.60
15.20 ± 5.07
3.56 ± 0.45a
0.78 ± 0.26
19.54 ± 5.09
14.26 ± 3.48
3.08 ± 1.09ab
0.76 ± 0.24
18.12 ± 4.77
Total free fatty acids
60.55 ± 16.69b
78.42 ± 7.80ab
92.14 ± 11.80ab
99.80 ± 11.60a
110.07 ± 21.8a
The values are mean ± standard deviation.
Sig., significance; on the same row, means with different letters differ significantly. Significance levels: ns, not significant; *p < 0.05; **p < 0.01, n = 6.
ns
*
**
*
ns
**
ns
*
ns
*
ns
**
ns
ns
*
194
S. Xiao et al. / Food Chemistry 121 (2010) 191–195
ham. Several authors have used activity measurement to study the
lipolysis in the subcutaneous adipose during the processing of drycured ham (Cava, Ferrer, Estévez, Morcuende, & Toldrá, 2004;
Motilva et al., 1993; Vestergaard et al., 2000). They indicated that
main lipases in the subcutaneous adipose are hormone-sensitive lipase (HSL) and lipoprotein lipase (LPL). However, this view is probably not correct because in adipose cell LPL is produced as a
secretary protein and acts at the vascular endothelium (Langfort,
Donsmark, Ploug, Holm, & Galbo, 2003; Wu, Olivecrona, & Olivecrona, 2003). Also, the conditions of adipose cells in ham would not
be compatible with the optimal conditions for the activation of LPL,
which requires pH 8.5. Therefore, we postulate that ATGL and HSL
are the key enzymes responsible for lipolysis during the ageing of
dry-cured ham.
The next question is which one (ATGL and HSL) is more important for lipolysis in dry-cured ham? Some studies have shown that
HSL had higher capacity than ATGL in hydrolysing triglycerides
in vitro (Jocken & Blaak, 2008; Mairal, Langin, Arner, & Hoffstedt,
2006). These researchers concluded that HSL is the major lipase
participating in the rate-limiting steps of lipolysis while ATGL is
responsible for basal lipolysis. In our study, the amount of FFAs increased dramatically at the first stage (0–3 months), while the
level of HSL protein remained relatively constant. This phenomenon could be another piece of evidence that support above conclusion: HSL is more important than ATGL for the lipolysis of adipose
tissues.
It has been well established that lipolytic enzymes are affected
by time/temperature cycles of each processing stage (Andrés, Cava,
Martín, Ventanas, & Ruiz, 2005; Motilva & Toldrá, 1993; Vestergaard et al., 2000). Table 2 indicated that during the first sampling
stage (0–3 months), the pH decreased significantly (6.06–5.76,
p < 0.01), and then remained relatively constant during most of
the ageing period before sharp decrease (43.0%) at the last phase.
It is well known that proteins precipitate if the pH is close to their
isoelectric point. A recent study (Arrese, Patel, & Soulages, 2006)
indicated that the isoelectric point of TG-lipase from adipose tissues is 5.8–6.0. Thus, the low pH at the later phase of ripening period (9–15 months) might be close to the isoelectric point of the two
lipases, which probably resulted in denaturation of the two lipases.
This can, in some degree, explain the decrease of HSL and ATGL
protein levels in later stages of ripening (Fig. 1). Also, the low pH
at the later stages of ripening would inactivate the two lipases.
Mairal et al. (2006) found that ATGL showed 50% less activity at
pH 6 than pH 7 and HSL showed an optimum activity at around
pH 7.0. These results indicated that the lower in pH, the lower
activity for the two lipases. After 15-months ageing, compared
with the first sampling stage (0 month), aw decreased dramatically
(0.8, p < 0.01, Table. 2) and NaCl concentration increased significantly (0.69%, p < 0.01, Table. 2). This indicates that hams experienced a relatively strong dehydration and salt penetration after
15 months of dry-curing. However, both aw and NaCl concentration
were slightly changed between 6 and 15 month ageing (aw, 0.82–
0.80; NaCl concentration, 0.65–0.69%). Two possible facts during
the processing of Xuanwei ham may help explaining the phenom-
ena. Firstly, we added vegetable oil on the surface of ham in order
to prevent water loss and extensive lipid oxidation during the later
stage of the ripening period. Secondly, there maybe relatively low
moisture loss during the 15 months ageing period at relatively
high relative humidity (58–80%) conditions. Similar conditions
were reported by Coutron-Gambotti and Gandemer (1999) who
worked on Corsican ham. It was reported that water activity inside
the dry-cured ham was important in controlling the enzyme activity, especially when aw approached to 0.9 or lower (Toldrá, 2006;
Vestergaard et al., 2000). It is indubitable that enzymes are usually
more soluble in dilute salt solutions than in pure water. When the
salt concentration is high enough, the proteins will be sufficiently
dehydrated to lose solubility. These may be another reason contributing to the decrease of HSL and ATGL protein levels as the ripening time progressed. These findings agreed with the reports of
Toldrá et al. (1997) who reported that lipase activity was inhibited
by curing agents especially salt, which played an important role in
controlling the enzyme activities.
In this study, we used Western blotting to measure HSL and
ATGL protein levels instead of activity measurement. The major
drawback for activity measurement is that it can only detect a
group of enzymes (e.g. basic lipase, acid lipase or neural lipase)
not the specific lipases. Although we have not measured the activity of these two lipases, this study can help better understanding
the lipases profile in dry-cured ham especially for ATGL, which is
a newly identified enzyme for lipolysis. The results showed that
ATGL and HSL could be detected during the 15 months of ripening
period. Further research is needed to develop appropriate methods
to measure the activity of HSL and ATGL, and find the correlation
between lipase activity and flavour development in dry-cured
hams.
3.2. Preferential substrates of HSL and ATGL
Oleic acid (C18:1, ranging from 28.37 to 48.98 mg g 1), palmitic
acid (C16:0, ranging from 10.67 to 26.66 mg g 1), and linoleic acid
(C18:2, ranging from 9.47 to 14.26 mg g 1) were the major FFAs
produced during Xuanwei ham maturation (Table 1). This indicated that triacylglycerols containing these three fatty acids such
as POL, OOL and POO might be easier targets for lipolysis, and most
likely, they were preferred substrates for ATGL and HSL.
Until now, little has been known about the specificity of endogenous lipases of adipose tissues (Toldrá & Verplaetse, 1994), and the
main substrates for HSL and ATGL are not clearly known. However,
Coutron-Gambotti and Gandemer (1999) who conducted the work
on Corsican ham showed preferential losses of POL (palmitoyloleoyl-linoleoyl glycerol), POO (di-oleoyl-palmitoyl glycerol) and
OOL (di-oleoyl-linoleoyl glycerol) during processing. Additionally,
Hazel and Sidell (2003) reported that the rank order of substrate
preference for adipose tissue HSL was: polyunsaturates > monoenes > saturates, demonstrating that in vivo HSL might prefer
long-chain unsaturated fatty acids as the substrates. Thereby, it
contributed to the observed preferential mobilisation of some
highly unsaturated fatty acids.
Table 2
Changes in pH, aw and NaCl concentration in adipose tissue during Xuanwei ham ripening.A
Ripening time (months)
pH
aw
NaCl (%)
A
B
0
3
6
9
15
Sig.B
6.06 ± 0.06a
0.89 ± 0.021a
0.55 ± 0.058b
5.76 ± 0.16bc
0.88 ± 0.014ab
0.55 ± 0.027b
5.71 ± 0.12bc
0.82 ± 0.019ab
0.65 ± 0.04a
5.82 ± 0.11b
0.80 ± 0.014b
0.63 ± 0.035ab
5.57 ± 0.15c
0.80 ± 0.020b
0.69 ± 0.019a
**
The values are mean ± standard deviation.
Sig., significance; on the same row, means with different letters differ significantly. Significance levels: ns, not significant; **p < 0.01; *p < 0.05; n = 6.
**
*
S. Xiao et al. / Food Chemistry 121 (2010) 191–195
We speculate that TAGs containing significant proportions of
unsaturated fatty acids are the main substrates of HSL and ATGL.
Such phenomena could also be attributed to the physical state of
these TAGs (e.g. POL, POO, OOL, etc.), which are liquid at the temperature (about 20 °C) during the ripening of dry-cured ham. This
water–oil interface circumstance is probably easier for the activation lipases, while the TAGs containing two saturated fatty acids
such as PSO and PPO are solid limiting the activation of lipases
(Coutron-Gambotti & Gandemer, 1999).
4. Conclusions
ATGL and HSL are the main lipases responsible for lipolysis during Xuanwei ham maturation. They could be detected even after
15 months of ripening, suggesting that both ATGL and HSL possibly
play important roles in FFAs generation during ham processing, but
HSL was more important than ATGL for adipose tissues lipolysis.
Acknowledgements
We express our gratitude to Dongheng Group, Yunnan Province,
China for providing samples. This work was supported by the National Project fund titled with ‘‘Development of Technologies on
Improving Food Attributes in Gel Forming and in Flavour” from
the Ministry of Science and Technology of China (2006BAD05A03).
References
Andrés, A. I., Cava, R., Martín, D., Ventanas, J., & Ruiz, J. (2005). Lipolysis in dry-cured
ham: Influence of salt content and processing conditions. Food Chemistry, 90,
523–533.
AOAC. (1999). Official methods of analysis (sixteenth ed.). Washington DC:
Association of Official Analytical Chemists.
Arrese, E. L., Patel, R. T., & Soulages, J. L. (2006). The main triglyceride-lipase from
the insect fat body is an active phospholipase A1: Identification and
characterization. Journal of Lipid Research, 47, 2656–2667.
Belfrage, P., Frederikson, G., Strälfors, P., & Tornqvist, H. (1984). Adipose tissue
lipases. In B. Borgström & H. L. Brockerman (Eds.), Lipase (pp. 365–416).
Amsterdam: Elsevier.
Cava, R., Ferrer, J. M., Estévez, M., Morcuende, D., & Toldrá, F. (2004). Composition
and proteolytic and lipolytic enzyme activities in muscle Longissimus dorsi from
Iberian pigs and industrial genotype pigs. Food Chemistry, 88, 25–33.
Coutron-Gambotti, C., & Gandemer, G. (1999). Lipolysis and oxidation in
subcutaneous adipose tissue during dry-cured ham processing. Food
Chemistry, 64, 95–101.
Folch, J., Lees, M., & Stanley, G. H. S. (1957). A simple method for the isolation and
purification of total lipids from animal tissues. Journal of Biochemistry, 266,
497–509.
Gandemer, G., Morvan-Mahi, B., Meynier A., & Lepercq, M. (1991). Quantitative and
qualitative analysis of free fatty acids in meat products using ion exchange
resin. In Proceedings of the 37th congress of meat science and technology,
Kulmbach (pp. 1139–1142).
Granneman, J. G., Moore, H. H., Granneman, R. L., Greenberg, A. S., Obin, M. S., & Zhu,
Z. X. (2006). Analysis of lipolytic protein trafficking and interactions in
adipocytes. Journal of Biological Chemistry, 282(8), 5726–5735.
195
Hazel, J. R., & Sidell, B. D. (2003). The substrate specificity of hormone-sensitive
lipase from adipose tissue of the Antarctic fish Trematomus newnesi. The Journal
of Experimental Biology, 207, 897–903.
Jenkins, C. M., Mancuso, D. J., Yan, W., Sims, H. F., Gibson, B., & Gross, R. W. (2004).
Identification, cloning, expression, and purification of three novel human
calcium-independent phospholipase A2 family members possessing
triacylglycerol lipase and acylglycerol transacylase activities. Journal of
Biological Chemistry, 279, 48968–48975.
Jocken, J. W. E., & Blaak, E. E. (2008). Catecholamine-induced lipolysis in adipose
tissue and skeletal muscle in obesity. Physiology and Behavior, 94, 219–230.
Langfort, J., Donsmark, M., Ploug, T., Holm, C., & Galbo, H. (2003). Hormone-sensitive
lipase in skeletal muscle: Regulatory mechanisms. Acta Physiology of
Scandinavia, 178, 397–403.
Mairal, A., Langin, D., Arner, P., & Hoffstedt, J. (2006). Human adipose triglyceride
lipase (PNPLA2) is not regulated by obesity and exhibits low in vitro triglyceride
hydrolase activity. Diabetologia, 49, 1629–1636.
Marra, A. I., Salgado, A., Prieto, B., & Carballo, J. (1999). Biochemical characteristics
of dry-cured lacón. Food Chemistry, 67, 33–37.
Martín, D., Ruiz, J., Flores, M., & Toldrá, F. (2006). Effect of dietary conjugated linoleic
acid (CLA) on pig muscle and adipose tissue lipase and esterase activities.
Journal of Agricultural and Food Chemistry, 54, 9241–9247.
Motilva, M. J., Toldrá, F., Aristoy, M. C., & Flores, J. (1993). Subcutaneous adipose
tissue lipolysis in the processing of dry-cured ham. Journal of Food Biochemistry,
16, 323–335.
Motilva, M. J., & Toldrá, F. (1993). Effect of curing agents and water activity on pork
muscle and adipose subcutaneous tissue lipolytic activity. Lebensmittel
Untersuchung und-Forschung, 196, 228–231.
Schweiger, M., Schreiber, R., Haemmerle, G., Lass, A., Fledelius, C., Jacobsen, P., et al.
(2006). Adipose triglyceride lipase and hormone-sensitive lipase are the major
enzymes in adipose tissue triacylglycerol catabolism. Journal of Biochemistry,
281, 40236–40241.
Shen, W. J., Patel, S., Yu, Z. X., Jue, D., & Kraemer, F. B. (2007). Effect of rosiglitazone
and high fat diet on lipase/esterase expression in adipose tissue. Biochimica et
Biophysica Acta, 1771, 177–184.
Toldrá, F., & Verplaetse, A. (1994). Endogenous activity and quality for raw product
processes. In Proceedings of the 40th congress of meat science and technology, The
Hague (pp. 41–53).
Toldrá, F., Flores, M., & Sanz, Y. (1997). Dry-cured ham flavour: Enzymatic
generation and process influence. Food Chemistry, 59, 523–530.
Toldrá, F. (1998). Proteolysis and lipolysis in flavour development of dry-cured meat
products. Meat Science, 49, S101–S110.
Toldrá, F., & Flores, M. (1998). The role of muscle proteases and lipases in flavour
development during the processing of dry-cured ham. CRC Critical Reviews in
Food Science and Nutrition, 38, 331–352.
Toldrá, F. (2006). The role of muscle enzymes in dry-cured meat products with
different drying conditions. Trends in Food Science and Technology, 17, 164–168.
Vestergaard, C. S., Schivazappa, C., & Virgili, R. (2000). Lipolysis in dry-cured ham
maturation. Meat Science, 55, 1–5.
Villena, J. A., Roy, S., Sarkadi-Nagy, E., Kim, K. H., & Sul, H. S. (2004). Desnutrin, an
adipocyte gene encoding a novel patatin domain-containing protein, is induced
by fasting and glucocorticoids: Ectopic expression of desnutrin increases
triglyceride hydrolysis. Journal of Biochemistry, 279, 47066–47075.
Wood, J. D., Enser, M., Fisher, A. V., Nute, G. R., Sheard, P. R., Richardson, R. I., et al.
(2007). Fat deposition, fatty acid composition and meat quality: A review. Meat
Science, 78, 343–358.
Wu, Gengshu, Olivecrona, Gunilla, & Olivecrona, Thomas (2003). The distribution of
lipoprotein lipase in rat adipose tissue. The Journal of Biological Chemistry, 278,
11925–11930.
Zhou, G. H., & Zhao, G. M. (2007). Biochemical changes during processing of
traditional Jinhua ham. Meat Science, 77, 114–120.
Zimmermann, R., Strauss, J. G., Haemmerle, G., Schoiswohl, G., Birner-Gruenberger,
R., Riederer, M., et al. (2004). Fat mobilization in adipose tissue is promoted by
adipose triglyceride lipase. Science, 306, 1383–1386.