University of California, Irvine- Institutional Review Board
advertisement

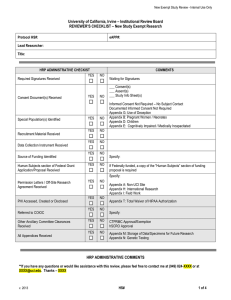
New Study Review - Internal Use Only University of California, Irvine- Institutional Review Board REVIEWER’S CHECKLIST – New Study Protocol HS#: eAPP#: Tabled Resubmission Expedited Full Committee Lead Researcher: Title: HRP ADMINISTRATIVE CHECKIST YES Required Signatures Received Consent Document(s) Received YES COMMENTS NO NO YES NO YES NO YES NO YES NO Human Subjects section of Federal Grant Application/Proposal Received YES NO Copy of DHHS-approved Consent document and Protocol Received YES NO Permission Letters / Off-Site Research Agreement Received YES NO YES NO Special Population(s) Identified Recruitment Material Received Data Collection Instrument Received Source of Funding Identified Waiting for Signatures ___ Consent(s) ___ Assent(s) ___ Study Info Sheet(s) Appendix G (&O): Use of Deception Appendix O: Waiver or Alteration of Informed Consent Appendix P: Waiver of Written Informed Consent Appendix Q: Use of Short Form Consent Appendix B: Pregnant Women / Neonates Appendix C: Prisoners Appendix D: Children Appendix E: Cognitively Impaired / Medically Incapacitated Specify: If Federally funded, a copy of the "Human Subjects” section of funding proposal is required If DHHS funded and not investigator initiated, this is required Specify: PHI Accessed, Created or Disclosed Referred to COIOC Referred for Scientific Review Other Ancillary Committee Clearances Received v. 2013 Appendix A: Non-UCI Site Appendix H: International Research Appendix I: Field Work ___ HIPAA Authorization Form(s) received Appendix T: Partial Waiver of HIPAA Authorization Appendix T: Total Waiver of HIPAA Authorization YES NO YES NO YES NO Specify: Date: CTPRMC Approval/Exemption hSCRO Approval IBC Approval RSC Approval HS# 1 of 5 New Study Review - Internal Use Only Master (Sponsor) Protocol Received Drug / Biologic or Medical Device Identified Investigator’s Brochure Received All Appendices Received YES NO YES NO YES NO YES NO Version: Appendix J: Drug / Biologic Study Appendix K: Device Study Version: Appendix L: Use of Placebo or Sham Procedure Appendix M: Storage of Data/Specimens for Future Research Appendix N: Genetic Testing Appendix S: Data Safety Monitoring Plan HRP ADMINISTRATIVE COMMENTS **If you have any questions or would like assistance with this review, please feel free to contact me at (949) 824-XXXX or at XXXX@uci.edu. Thanks – XXXX ADMINISTRATIVE QUESTIONS AND NOTES FOR THE IRB Notes for the IRB: Questions for the IRB: 1. Question X? YES NO (Please comment as necessary): 2. Appendix T: A partial waiver of HIPAA for recruitment purposes is requested. Please review Appendix T. In order to waive HIPAA Authorization, the IRB must determine that the study meets all of the following criteria: The use or disclosure of PHI involves no more than minimal risk Granting of the waiver will not adversely affect privacy rights and welfare of the individuals whose records will be used The project could not practicably be conducted without a waiver The project could not practicably be conducted without use of PHI The privacy risks are reasonable relative to the anticipated benefits of research An adequate plan to protect identifiers from improper use and disclosure is included in the research proposal An adequate plan to destroy the identifiers at the earliest opportunity, or justification for retaining identifiers, is included in the research proposal The project plan includes written assurances that PHI will not be re-used or disclosed for other purposes Whenever appropriate, the subjects will be provided with additional pertinent information after participation Is the request for a partial waiver acceptable? YES v. 2013 HS# 2 of 5 New Study Review - Internal Use Only NO (Please comment as necessary): ADMINISTRATIVE COMMENTS FOR THE LEAD RESEARCHER (LR) UCI IRB REVIEWER’S CHECKLIST A. Criteria for IRB Review and Approval: Please review the federal criteria for IRB approval and indicate whether the research meets each criterion by checking the appropriate box. List any concern that you would like communicated to the researcher in the corresponding comment box or in the open space below. (Criteria for IRB approval of research in accordance with 45 CRF 46.111, 21 CFR 56.111 and UCI Policy) CRITERIA FOR IRB REVIEW AND APPROVAL 1 The IRB has the expertise needed to review this research. 2 I, the IRB reviewer, have a conflicting interest with this protocol. 3 The statement of purpose/hypothesis is adequate. 4 Study personnel appear appropriate and qualified. COMMENTS YES NO YES NO YES NO YES NO If no, contact IRB staff to arrange consultation with expert. If yes, contact HRP staff ASAP to arrange for reassignment of this protocol. Risk/Benefit Assessment – Risks include possible physical, psychological, economic, social and legal harms. Risks to subjects are minimized by using procedures YES NO 5 which are consistent with sound research design and which do not unnecessarily expose subjects to risk. Risks to subjects are minimized, whenever appropriate, YES NO N/A 6 by using procedures already being performed on the subjects for diagnostic or treatment purposes. Risks to subjects are reasonable in relation to both: N YES NO / anticipated benefits, if any, to subjects; and 7 the importance of the knowledge that may reasonably be expected to result. Subject Selection Selection of subjects is equitable in relation to the YES NO 8 purposes of the research and the setting in which the research will be conducted. Selection of subjects (i.e., inclusion/exclusion criteria) is YES NO 9 appropriate based on the research and the setting in which the research will be conducted. YES NO N/A The recruitment process minimizes the potential for 10 undue influence or coercion. 11 v. 2013 Compensation - neither the amount of payment nor the proposed method and timing of disbursement is coercive or presents potential for undue influence. YES HS# NO N/A 3 of 5 New Study Review - Internal Use Only 12 Recruitment materials are appropriate. Informed Consent Informed consent is sought from each prospective subject or the subject's legally authorized representative and 13 appropriately documented in accordance with, and to the extent required by 45 CFR 46.116 and 45 CFR 46.117, and 21 CFR 50.25 and 21 CFR 50.27 as applicable. Subject Protections The research plan makes adequate provision for monitoring the data collected to ensure the safety of subjects. 14 For > minimal risk studies, UCI requires investigators conducting clinical investigations to at a minimum, have a DSM plan. YES NO N/A YES NO N/A YES NO N/A 15 The research plan makes adequate provisions to protect the privacy of subjects. YES NO 16 The research plan makes adequate provisions to maintain the confidentiality of data. YES NO YES NO YES NO 17 The research does involve subjects likely to be vulnerable to coercion or undue influence, such as: children, prisoners, pregnant women, mentally disabled persons, or economically / educationally disadvantaged persons. If YES, the research plan does include additional safeguards to protect their rights and welfare. B. Risk Assessment: Virtually no risk [Exempt Registration] If Virtually No Risk, indicate all corresponding Category(ies): No more than Minimal Risk [Expedited review] If Minimal Risk, indicate all corresponding Category(ies): Greater than Minimal Risk [Full Committee review] Please provide a rationale for any change in the risk assessment (e.g., from Expedited to Full Committee or vice versa). C. IRB Recommendation: Approve Minor Changes v. 2013 Tabled to Full Committee Send a Copy of Tabled Memo to Department Chair Tabled to Subcommittee Send a Copy of Tabled Memo to Department Chair HS# 4 of 5 New Study Review - Internal Use Only Disapprove Please provide a rationale below if recommendation is to restrict or disapprove the research. D. IRB Review cycle: 12 months 6 months* 3 months* 3 years Other*: _______________ * Please provide a rationale below if recommended review cycle is less than 12 months. E. Reviewer Comments: Reviewer’s Signature Date Note: The information provided on this form may be preliminary and may not necessarily reflect the discussion and final decision and/or recommendation of the Committee. v. 2013 HS# 5 of 5