Appendix S DESCRIPTION OF DATA SAFETY MONITORING PLAN (DSMP)

Lead Researcher:
Study Title:
HS #:
Appendix S
DESCRIPTION OF DATA SAFETY MONITORING PLAN (DSMP)
University of California, Irvine
Institutional Review Board
THE COMPLETION OF APPENDIX S IS REQUIRED
FOR ALL CLINICAL INVESTIGATIONS
Researchers may cut and paste into Appendix S from the following sources, as applicable:
Please read the applicable HRP webpage for information about data and safety monitoring plans.
All clinical investigations, including Phase I, II and III clinical studies, involving greater than minimal risk to participants are, at a minimum, required to develop a plan to assure the safety and welfare of the research participants.
Please answer all of the following:
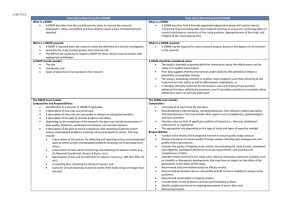
1. In an effort to ensure that the Data Safety Monitoring Board (DSMB) is appropriately constituted, describe the composition of the Board, including the relevant experience of the
Board (for UCI initiated studies: include the name, title and experience of the individual(s)):
For NIH-sponsored clinical trials, the DSMP should be part of the grant application
For “for-profit” sponsor-initiated clinical trials, a FDA-approved DSMP should be part of the Master Protocol.
For studies conducted at the Institute for Clinical and Translational Science
(ICTS) or Cancer Center (CTPRMC), the DSMP approved by one of these committees should be inserted into this appendix.
2. Indicate how frequently the DSMB will review and evaluate the accumulated study data for participant safety, study conduct and progress, and, when appropriate, efficacy.
3. Explain the process by which the DSMB will make recommendations concerning the continuation, modification, or termination of the trial.
4. Describe the event(s) that would trigger an unscheduled review. Also include stopping guidelines and un-blinding rules, if applicable.
5. List who will be locally monitoring and collecting information on adverse events and/or unanticipated problems (e.g., UCI Lead Researcher, Research Coordinator, etc.). Include the name, title and experience of the individual(s).
6. D escribe the plan for annual reporting of the participants’ safety, and the study’s conduct, progress, and efficacy, when appropriate. Note: At the Continuing Review, provide documentation that the DSMB met as described above and indicate any findings or recommendations made. For UCI initiated studies, provide a copy of the annual report at the time of continuing review.
IRB version 03-17-08