382
Auxin signalling: the beginning, the middle and the end
Ottoline Leyser
The plant hormone auxin is central to the regulation of growth
and development. Recent work has demonstrated that auxin
signalling depends on targeted protein degradation, and in the
past year this model has been strengthened. The focus is now
on identifying the targets of this degradative pathway,
determining how auxin influences the degradative process and
linking the turnover of specific proteins to the numerous
downstream responses to auxin.
Addresses
Department of Biology, University of York, Heslington,
York YO10 5YW, UK; e-mail: hmol1@york.ac.uk
Current Opinion in Plant Biology 2001, 4:382–386
1369-5266/01/$ — see front matter
© 2001 Elsevier Science Ltd. All rights reserved.
Abbreviations
ABP1
AUXIN BINDING PROTEIN 1
ARE
auxin response element
ARF
auxin response factor
PID
PINOID
PIN
PIN-FORMED
SCF
SKP1, Cullin/CDC53, F-box protein
Introduction
The auxin signal is perceived by plant cells and rapidly
transduced into a wide variety of responses in growth and
development. These include changes in the direction of
growth, changes in shoot and root branching and changes
in vascular differentiation. Our current understanding of the
events that connect auxin to these diverse responses is very
patchy. To paraphrase a famous Monty Python sketch in
which a scientist is coaxed into revealing her earth-shattering
theory about the brontosaurus, our understanding of auxin
signalling is very very thin at one end, very very fat in the
middle, and very very thin at the other end. This is, of
course, rather an exaggeration but, nonetheless, we appear
to know a bit more about the middle section of the auxin
signalling pathway than about what goes on at either end.
This review discusses recent developments in understanding
this central section and its connections upstream to auxin
and downstream to auxin responses.
Auxin and protein stability
Over the past few years, the molecular analysis of auxin
response mutants of Arabidopsis thaliana has led to a model in
which auxin signalling is mediated by regulated protein
degradation. This work has been recently reviewed elsewhere ([1,2]; Figure 1). In brief, wild-type auxin sensitivity
depends on the function of a protein ubiquitin ligase
complex of the SCF type. SCF complexes are found
throughout the eukaryotes and consist of a SKP1 homologue,
a Cullin/CDC53 homologue, an F-box protein and an
RBX1/ROC1 homologue. Combining data from a variety of
systems indicates that the RBX1/ROC1 protein, which has a
RING-H2 finger domain (common among polyubiquitin
synthesising proteins), acts together with Cullin/CDC53 to
catalyse the synthesis of ubiquitin polymers [3]. Cullin/
CDC53 interacts with RBX1/ROC1 through its carboxyl
terminus [3]. The Cullin/CDC53 carboxyl terminus is also
required for nuclear localisation and can be modified
by the conjugation of a ubiquitin-like protein of the
NEDD8/RUB1 family [4]. NEDD8/RUB1 conjugation cannot occur without nuclear localisation and appears to increase
the activity of the SCF complex [4,5]. The Cullin/CDC53
amino terminus interacts with SKP1, which acts as a scaffold
to bring together the dimer formed by RBX1/ROC1 and
Cullin/CDC53 with the F-box protein [3]. F-box proteins
form a diverse family defined by the amino-terminal F-box,
which mediates its interaction with SKP1 [6,7]. The carboxyl
terminus of F-box proteins consists of one of a variety of
protein–protein interaction domains, and it is this domain
that is responsible for the recruitment of specific substrates
to the SCF complex where they are ubiquitinated and
targeted for degradation by the 26S proteasome.
The F-box protein determines which proteins are degraded
and, consequently, SCF complexes can be considered to
be the hub of the degradative machinery. Three substrates
feed into this complex: activated ubiquitin and activated
NEDD8/RUB1 (via their respective activating enzymes
and conjugating enzymes), and the target proteins destined
for degradation (Figure 1).
Arabidopsis mutants have been recovered that are defective
in several components of this system, and these share a
suite of morphological phenotypes that reflect their
reduced sensitivity to auxin. These mutants include axr1,
which is defective in RUB1 activation [8,9], and tir1, which
has a mutation in a gene encoding an F-box protein [10,11].
Neither of these mutations is likely to block the functioning
of the auxin-related ubiquitination pathway completely.
By analogy with other systems, blocking RUB1 conjugation
is likely only to reduce the activity of SCFTIR1 [12];
furthermore, there are several F-box proteins in the
Arabidopsis genome that are similar to TIR1, and hence
TIR1 could be partially functionally redundant [7]. This
partial loss of SCF function in axr1 and tir1 mutants is
supported by the observation that loss of AXR1 or TIR1
results in a similar phenotype, but the axr1 phenotype is
more severe than that conferred by tir1, whereas the
double mutant combination has synergistic effects [10].
Targets for the AXR1/TIR1 pathway
Mounting, although as yet unpublished, data suggest that
targets for the AXR1/TIR1 degradative pathway might
include the Aux/IAA proteins. Aux/IAA genes are found
throughout the plant kingdom and were first identified
because many of them are rapidly induced as a primary
Auxin signalling: the beginning, the middle and the end Leyser
383
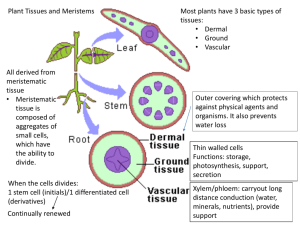
Figure 1
AUXIN
Auxin efflux
carrier
Auxin
dependent?
Changes in auxin transport
Ubiquitin
Activation
Conjugation
Ligation
Ubiquitin
Degradation
Target
proteins
RBX1/
ROC1
Responses
SCF complex
Auxin
regulated?
Cullin/
CDC53
F-box protein
SKP1
Changes in gene expression
Auxin
dependent?
II
NEDD8/
RUB1
Activation
and conjugation
NEDD8/
RUB1
Aux/IAA
ARF
IV
III
IV
III
I
DBD
ARE
Auxin-responsive gene
Current Opinion in Plant Biology
SCF complex components (grey shading) and their probable
involvement with potential target proteins that are conjugated to
ubiquitin and degraded during auxin signalling. Degradation of auxin
efflux carriers would influence auxin distribution, and degradation of
Aux/IAA proteins would influence the gene expression of auxinresponsive genes. DBD, DNA-binding domain.
response to auxin [13]. These genes can also be induced by
cycloheximide, indicating that they are kept inactive by a
rapidly turned-over repressor that is in some way inactivated
by auxin [14]. Aux/IAA genes encode short-lived nuclear
proteins that share four highly conserved domains [13,15].
Domains III and IV allow homodimerisation and heterodimerisation within the Aux/IAA family, and also between
Aux/IAA proteins and members of the auxin response factor
(ARF) family [16,17]. ARFs bind to auxin response elements
(AREs) in the promoters of auxin-inducible genes through a
carboxy-terminal DNA-binding domain, and this binding
appears to be auxin-independent [17,18]. ARFs contain
domains that are homologous to domains III and IV of the
Aux/IAA proteins, allowing dimerisation within the ARF
family as well as between ARF family and Aux/IAA family
members [16]. The functional significance of ARFs in auxin
signalling has been confirmed by the identification of
mutations in ARF genes that confer auxin-related phenotypes such as reduced tropic growth and embryo patterning
defects, as well by reduced expression of auxin-inducible
genes including those encoding Aux/IAAs [19,20•].
This is most probably because dimerisation with an
Aux/IAA protein prevents dimerisation with a second ARF
(Figure 1). Although the transcription of ARF genes and
the DNA binding of ARF proteins is not changed by auxin,
the transcription of Aux/IAA proteins is. Therefore,
Aux/IAA proteins appear to be central to the auxin response,
mediating downstream auxin responses by regulating gene
activity through interactions with ARFs. As the promoters
of many Aux/IAA genes themselves contain ARF-binding
sites, Aux/IAA proteins presumably feed back to regulate
their own transcription. In this respect, it is interesting to
note that a characteristic property of Aux/IAA proteins is
their short half-lives, some of which are the shortest
recorded for any protein being in the order of eight
minutes [15,22••]. This raises the possibility that Aux/IAA
proteins are the short-lived repressors of their own transcription, as implicated by the cycloheximide experiments
described above.
ARFs regulate transcription as ARF–ARF dimers [17].
Overexpression of Aux/IAA proteins can block auxininducible transcription from an ARF-activated promoter [21].
The instability of Aux/IAA proteins is required for normal
auxin signalling. Semi-dominant mutations in the conserved
domain II of several Aux/IAA family members have
been recovered and found to increase the stability of the
proteins and to confer a range of auxin-related phenotypes,
including changes in the transcription of a range of
384
Cell signalling and gene regulation
Aux/IAA genes [22••,23••,24–28]. These data support the
hypothesis that Aux/IAA proteins regulate their own
transcription. Dimerisation is also essential for Aux/IAA
function because mutations in domain III that prevent
dimerisation can suppress the effects of intragenic domain-II
mutations [22••].
A second protein family that could include targets for
auxin-regulated degradation is the PIN-FORMED (PIN)
family of auxin efflux carriers ([29••]; Figure 1). These
efflux carriers are responsible for the active and directional
transport of auxin through plant tissues. The ability to
pump auxin directionally in this way is required for tropic
growth, regulation of root and shoot branching, and developmental patterning [30–34]. The distribution of one PIN
family member, EIR1/PIN2/AGR1, has been shown to be
regulated post-translationally [29••]. Accumulation of the
protein is increased in the axr1 mutant background and
reduced in response to auxin addition. These data suggest
a molecular mechanism for the long-recognised feedback
between auxin signalling and auxin transport [35].
Further upstream, the site of auxin perception is still a
matter of considerable debate. The best candidate auxin
receptor is AUXIN BINDING PROTEIN 1 (ABP1).
ABP1 was first identified because of its ability to bind
auxin specifically and with physiologically relevant affinity
(reviewed in [39]). Most ABP1 is retained in the endoplasmic reticulum but a small amount appears to escape and
act at the cell surface [40]. There is now good evidence
that ABP1 mediates downstream auxin responses.
Expression of ABP1 in transgenic cell lines and plants can
alter auxin sensitivity in a variety of responses, including
cell expansion [41] and guard-cell potassium currents [42].
More recently, an insertion in the Arabidopsis ABP1 gene
has been identified, allowing analysis of the null mutant
phenotype for the first time [43•]. Plants that are homozygous for this insertion die in the early globular phase of
embryogenesis. This phenotype certainly demonstrates
that ABP1 is an essential gene but further analysis will be
required to determine whether binding of auxin to ABP1 is
linked to the proteolytic pathway, the Aux/IAA proteins or
the auxin efflux carriers.
The head and tail of the brontosaurus
These findings have led to a model in which auxin controls
the abundance of the Aux/IAA transcriptional regulators,
the auxin efflux carriers and quite possibly a range of other
proteins (Figure 1). This is an attractive model because it
provides an explanation as to how a single simple molecule
can induce such diverse effects on different plant tissues.
The specificity of auxin response is not in the auxin molecule itself but in the available targets for degradation,
which are determined by cell type. The current model is,
however, seriously deficient in two respects: the upstream
inputs and the downstream outputs for this pathway are
largely unknown.
We are at present entirely ignorant of how auxin influences
the activity of the degradative machinery. There are two
possibilities: either auxin could change the activity of the
SCF complex or it could regulate the ability of target
proteins to be recruited to it. In other SCF systems,
recruitment of targets is dependent on their phosphorylation status [6]. It is, therefore, possible that auxin acts to
regulate the phosphorylation of degradation targets.
Certainly auxin-regulated kinase activities have been
identified [36]; furthermore, genetic analysis has identified
kinases and phosphatases that are required for normal
auxin responses. These include the PINOID (PID) serine/
threonine kinase, loss of which results in altered auxin
responses [37•], and the RCN1 PP2A-type phosphatase
subunit that is required for normal auxin-regulated elongation responses [38]. The phosphorylation status of SCF
targets also provides a possible point for the integration of
signals. Interestingly, it has recently been shown that Aux/IAA
proteins can be phosphorylated by the photoreceptor
phytochrome A [24]. One way in which this phosphorylation
could influence Aux/IAA activity is through changes in
protein stability.
There is considerable evidence for multiple sites of auxin
perception and there is no reason to suppose that ABP1 is
the only auxin receptor. Additional possible receptors include
the auxin efflux carriers themselves. In this context, it is
interesting to note that glucose perception in yeast is
mediated by a receptor with homology to glucose transporters via an SCF-dependent proteolytic pathway [44].
Events downstream of the potential SCFTIR1 targets are
also unclear. Changes in the stability of various Aux/IAA
proteins result in a gene-specific pattern of morphological
phenotypes, such as changes in root and shoot branching,
cell elongation defects, light responses and defects in tropic
growth [25–28]. How these phenotypes arise is unclear.
There is good evidence that Aux/IAA proteins regulate
their own transcription but additional genes in this regulatory loop are comparatively uncharacterised. This reflects
a widespread problem in developmental biology in which
key transcriptional regulators are shown to regulate their
own transcription but further targets are harder to find.
Additional auxin-responsive genes have been identified
and many have AREs in their promoters, implicating ARFs
and Aux/IAAs in their regulation. The functional importance of some of these genes in mediating auxin responses
is now beginning to be established. For example, the
DFL1 gene of Arabidopsis influences auxin sensitivity and
stem elongation. DFL1 is encoded by a member of the
GH3 family of genes and, characteristically for this family,
it is auxin-inducible and has AREs in its promoter [45];
however, the biochemical function of GH3s is not clear.
More direct links have been forged between elongation
and the activity of the expansin family of cell wall proteins,
which catalyse cell wall loosening [46]. Links between
auxin and expansin expression are being uncovered in a
Auxin signalling: the beginning, the middle and the end Leyser
variety of systems. For example, addition of auxin and of
expansin protein to shoot apical meristems stimulates leaf
outgrowth [47,48•]. In situ hybridisation shows that
expansin transcripts accumulate at the presumptive sites of
leaf initiation and these expansins are auxin-inducible
[49,50]; however, it is likely that auxin can also regulate
expansin activity at the post-transcriptional level through
its effects on cell wall pH [46].
Conclusions
Progress in understanding auxin signalling has been
startling in recent years, resulting in a working model for its
mode of action. Furthermore, important advances in understanding the very earliest steps in auxin perception have
been made, providing tools to characterise the roles of ABP1
and PIN family members in auxin perception. Linking
these pathways to the diversity of auxin responses is now a
key challenge. A satisfying development here is the convergence of scientists working on auxin signalling with those
working on particular aspects of plant biology in which auxin
plays a role, resulting in a gradual linking of previously
disparate parts of the puzzle. I hope it will not be long
before our fragmented picture will be as dead as a dinosaur.
Update
Recent work has demonstrated that the COP9 signalosome
interacts with SCFTIR1 and is required for efficient auxin
signalling [51••]. Its role appears to be in RUB1 deconjugation, suggesting that conjugation–deconjugation cycles
may be important in SCF function.
Acknowledgement
I thank Stephen Day for critical reading of the manuscript.
References and recommended reading
Papers of particular interest, published within the annual period of review,
have been highlighted as:
• of special interest
•• of outstanding interest
1.
Gray WM, Estelle M: Function of the ubiquitin-proteasome
pathway in auxin response. Trends Biochem Sci 2000, 25:133-138.
2.
del Pozo JC, Estelle M: F-box proteins and protein degradation: an
emerging theme in cellular regulation. Plant Mol Biol 2000,
44:123-128.
3.
Wu K, Fuchs SY, Chen A, Tan PL, Gomez C, Ronai Z, Pan ZQ: The
β-TRCP-ROC1 E3 ubiquitin ligase utilizes two distinct
SCFHOS/β
domains within CUL1 for substrate targeting and ubiquitin
ligation. Mol Cell Biol 2000, 20:1382-1393.
4.
Furukawa M, Zhang YP, McCarville J, Ohta T, Xiong Y: The CUL1
C-terminal sequence and ROC1 are required for efficient nuclear
accumulation, NEDD8 modification, and ubiquitin ligase activity of
CUL1. Mol Cell Biol 2000, 20:8185-8197.
5.
Wu K, Chen A, Pan ZQ: Conjugation of Nedd8 to CUL1 enhances
the ability of the ROC1–CUL1 complex to promote ubiquitin
polymerization. J Biol Chem 2000, 275:32317-32324.
6.
Craig KL, Tyers M: The F-box: a new motif for ubiquitin dependent
proteolysis in cell cycle regulation and signal transduction. Prog
Biophys Mol Biol 1999, 72:299-328.
7.
Xiao WY, Jang JC: F-box proteins in Arabidopsis. Trends Plant Sci
2000, 5:454-457.
385
8.
del Pozo JC, Timpte C, Tan S, Callis J, Estelle M: The ubiquitinrelated protein RUB1 and auxin response in Arabidopsis. Science
1998, 280:1760-1763.
9.
del Pozo JC, Estelle M: The Arabidopsis cullin AtCUL1 is modified
by the ubiquitin-related protein RUB1. Proc Natl Acad Sci USA
1999, 96:15342-15347.
10. Ruegger M, Dewey E, Gray WM, Hobbie L, Turner J, Estelle M: The
TIR1 protein of Arabidopsis functions in auxin response and is
related to human SKP2 and yeast Grr1p. Genes Dev 1998,
12:198-207.
11. Gray WM, del Pozo JC, Walker L, Hobbie L, Risseeuw E, Banks T,
Crosby WL, Yang M, Ma H, Estelle M: Identification of an SCF
ubiquitin-ligase complex required for auxin response in
Arabidopsis thaliana. Genes Dev 1999, 13:1678-1691.
12. Lammer D, Mathias N, Laplaza JM, Jiang W, Liu Y, Callis J, Goebl M,
Estelle M: Modification of yeast Cdc53p by the ubiquitin-related
protein Rub1p affects function of the SCFCdc4 complex. Genes
Dev 1998, 12:914-926.
13. Abel S, Theologis A: Early genes and auxin action. Plant Physiol
1996, 111:9-17.
14. Koahiba T, Ballas N, Wong LM, Theologis A: Transcriptional
regulation of PS-IAA4/5 and PS-IAA6 early gene expression by
indoleacetic acid and protein synthesis inhibitors in pea (Pisum
sativum). J Mol Biol 1995, 253:396-413.
15. Abel S, Oeller PW, Theologis A: Early auxin-induced genes encode
short-lived nuclear proteins. Proc Natl Acad Sci USA 1994,
91:326-330.
16. Kim J, Harter K, Theologis A: Protein–protein interactions among
the Aux/IAA proteins. Proc Natl Acad Sci USA 1997,
94:11786-11791.
17.
Ulmasov T, Hagen G, Guilfoyle TJ: Dimerization and DNA binding of
auxin response factors. Plant J 1999, 19:309-319.
18. Ulmasov T, Hagen G, Guilfoyle TJ: Activation and repression of
transcription by auxin-response factors. Proc Natl Acad Sci USA
1999, 96:5844-5849.
19. Hardtke CS, Berleth T: The Arabidopsis gene MONOPTEROS
encodes a transcription factor mediating embryo axis formation
and vascular development. EMBO J 1998, 17:1405-1411.
20. Harper RM, Stowe-Evans EL, Luesse DR, Muto H, Tatematsu K,
•
Watahiki MK, Yamamoto K, Liscum E: The NPH4 locus encodes the
auxin response factor ARF7, a conditional regulator of differential
growth in aerial Arabidopsis tissue. Plant Cell 2000, 12:757-770.
The authors of this paper establish causative links between ARF7 function,
auxin-regulated differential growth responses and Aux/IAA gene expression.
21. Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ: Aux/IAA proteins
repress expression of reporter genes containing natural and
highly active synthetic auxin response elements. Plant Cell 1997,
9:1963-1971.
22. Ouellet F, Overvoorde PJ, Theologis A: IAA17/AXR3: biochemical
•• insight into an auxin mutant phenotype. Plant Cell 2001,
13:829-841.
As the title suggests, this paper provides evidence for the biochemical basis
of the phenotypes conferred by mutations in the gene encoding
IAA17/AXR3. Dominant domain-II mutations are found to stabilise the
protein without affecting its subcellular localisation. Intragenic revertants of
the domain-II mutation are found to affect Aux/IAA dimerisation.
23. Worley CK, Zenser N, Ramos J, Rouse D, Leyser O, Theologis A,
•• Callis J: Degradation of Aux/IAA proteins is essential for normal
auxin signalling. Plant J 2000, 21:553-562.
Using protein fusions to the reporter protein luciferase in a protoplast assay,
Aux/IAA domain II was found to be a transferable protein-destabilisation tag.
24. Colon-Carmona A, Chen DL, Yeh KC, Abel S: Aux/IAA proteins are
phosphorylated by phytochrome in vitro. Plant Physiol 2000,
124:1728-1738.
25. Rouse D, Mackay P, Stirnberg P, Estelle M, Leyser O: Changes in
auxin response from mutations in an AUX/IAA gene. Science
1998, 279:1371-1373.
26. Tian Q, Reed JW: Control of auxin-regulated root development by
the Arabidopsis thaliana SHY2/IAA3 gene. Development 1999,
126:711-721.
386
27.
Cell signalling and gene regulation
Nagpal P, Walker LM, Young JC, Sonawala A, Timpte C, Estelle M,
Reed JW: AXR2 encodes a member of the Aux/IAA protein family.
Plant Physiol 2000, 123:563-573.
28. Rogg LE, Lasswell J, Bartel B: A gain-of-function mutation in IAA28
suppresses lateral root development. Plant Cell 2001,
13:465-480.
29. Sieberer T, Seifert GJ, Hauser MT, Grisafi P, Fink GR, Luschnig C:
•• Post-transcriptional control of the Arabidopsis auxin efflux carrier
EIR1 requires AXR1. Curr Biol 2000, 10:1595-1598.
The EIR1/AGR/PIN2 auxin efflux carrier was found to be unstable in a
wild-type background, but stabilised in an axr1 mutant background. This
suggests that it might be a target for SCFTIR1 and provides a possible
mechanism for feedback between auxin signalling and auxin transport.
30. Chen RJ, Hilson P, Sedbrook J, Rosen E, Caspar T, Masson PH:
The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a
component of the polar-auxin-transport efflux carrier. Proc Natl
Acad Sci USA 1998, 95:15112-15117.
31. Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S,
Swarup R, Graham N, Inze D, Sandberg G, Casero PJ, Bennett M:
Auxin transport promotes Arabidopsis lateral root initiation. Plant
Cell 2001, 13:843-852.
32. Chatfield SP, Stirnberg P, Forde BG, Leyser O: The hormonal
regulation of axillary bud growth in Arabidopsis. Plant J 2000,
24:159-169.
33. Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J,
Benfey P, Leyser O, Bechtold N, Weisbeek P, Scheres B:
An auxin-dependent distal organizer of pattern and polarity in the
Arabidopsis root. Cell 1999, 99:463-472.
34. Steinmann T, Geldner N, Grebe M, Mangold S, Jackson CL, Paris S,
Gälweiler L, Palme K, Jürgens G: Coordinated polar localization of
auxin efflux carrier PIN1 by GNOM ARF GEF. Science 1999,
286:316-318.
35. Mattsson J, Sung ZR, Berleth T: Responses of plant vascular
systems to auxin transport inhibition. Development 1999,
126:2979-2991.
36. Mockaitis K, Howell SH: Auxin induces mitogenic activated protein
kinase (MAPK) activation in roots of Arabidopsis seedlings.
Plant J 2000, 24:785-796.
37. Christensen SK, Dagenais N, Chory J, Weigel D: Regulation of auxin
•
response by the protein kinase PINOID. Cell 2000, 100:469-478.
Loss of PINOID (PID) function results in phenotypes that are reminiscent of
those caused by blocking auxin transport, but auxin transport is relatively normal
in the pid mutants. This paper describes the molecular characterisation of the
PID protein and provides evidence that PID regulates auxin sensitivity.
38. Deruere J, Jackson K, Garbers C, Soll D, DeLong A: The
RCN1-encoded A subunit of protein phosphatase 2A increases
phosphatase activity in vivo. Plant J 1999, 20:389-399.
39. Napier RM: Towards an understanding of ABP1. J Exp Botany
1995, 46:1787-1795.
40. Leblanc N, David K, Grosclaude J, Pradier JM, Barbier-Brygoo H,
Labiau S, Perrot-Rechenmann C: A novel immunological approach
establishes that the auxin-binding protein, Nt-abp1, is an element
involved in auxin signaling at the plasma membrane. J Biol Chem
1999, 274:28314-28320.
41. Jones AM, Im KH, Savka MA, Wu MJ, DeWitt NG, Shillito R,
Binns AN: Auxin-dependent cell expansion mediated by
overexpressed auxin-binding protein 1. Science 1998,
282:1114-1117.
42. Bauly JM, Sealy IM, Macdonald H, Brearley J, Droge S, Hillmer S,
Robinson DG, Venis MA, Blatt MR, Lazarus CM, Napier RM:
Overexpression of auxin-binding protein enhances the sensitivity
of guard cells to auxin. Plant Physiol 2000, 124:1229-1238.
43. Chen JG, Ullah H, Young JC, Sussman MR, Jones AM: ABP1 is
•
required for organized cell elongation and division in Arabidopsis
embryogenesis. Genes Dev 2001, 15:902-911.
After many years of controversy surrounding the role of ABP1 in growth and
development, a mutant with complete loss of function of ABP1 is described
in this paper. The mutation results in early embryo lethality and will provide
an important tool for determining the relationship between ABP1 and the
other parts of the auxin signalling machinery.
44. Ozcan S, Dover J, Johnston M: Glucose sensing and signaling by
two glucose receptors in the yeast Saccharomyces cerevisiae.
EMBO J 1998, 17:2566-2573.
45. Nakazawa M, Yabe N, Ichikawa T, Yamamoto YY, Yoshizumi T,
Hasunuma K, Matsui M: DFL1, an auxin-responsive GH3 gene
homologue, negatively regulates shoot cell elongation and lateral
root formation, and positively regulates the light response of
hypocotyl length. Plant J 2001, 25:213-221.
46. Cosgrove DJ: Plant cell enlargement and the action of expansins.
BioEssays 1996, 18:533-540.
47.
Fleming AJ, McQueen-Mason S, Mandel T, Kuhlemeier C: Induction
of leaf primordia by the cell wall protein expansin. Science 1997,
276:1415-1418.
48. Reinhardt D, Mandel T, Kuhlemeier C: Auxin regulates the initiation
•
and radial position of plant lateral organs. Plant Cell 2000,
12:507-518.
This paper provides a dramatic demonstration of the role of auxin in leaf
morphogenesis. Beautiful scanning electron micrographs show the ability of
auxin to induce leaf outgrowth in a band below the shoot apex.
49. Reinhardt D, Wittwer F, Mandel T, Kuhlemeier C: Localized
upregulation of a new expansin gene predicts the site of leaf
formation in the tomato meristem. Plant Cell 1998, 10:1427-1437.
50. Caderas D, Muster M, Vogler H, Mandel T, Rose JKC, McQueenMason S, Kuhlemeier C: Limited correlation between expansin
gene expression and elongation growth rate. Plant Physiol 2000,
123:1399-1413.
51. Schwechheimer C, Serino G, Callis J, Crosby W, Lyapina S,
•• Deshaies RJ, Gray WM, Estelle M, Deng X-W: Interactions of the
COP9 signalosome with the E3 ubiquitin ligase SCF TIR1 in
mediating the auxin response. Science 2001, 292:1379-1382.
Partial loss of function of the COP9 signalosome subunit CSN5 was found
to result in auxin resistance, and to confer synergistic auxin-related phenotypes when combined with partial loss of AXR1 function. Furthermore,
SCFTIR1 and COP9 subunits were co-immunoprecipitated with anti-cullin
antibodies, indicating that they interact directly in vivo.