What Powers the Stars? In brief, nuclear reactions.
advertisement

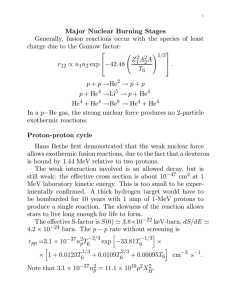
What Powers the Stars? In brief, nuclear reactions. But why not chemical burning or gravitational contraction? Bright star Regulus (& Leo dwarf galaxy). Nuclear Energy. Basic Principle: conversion of mass to energy according to Einstein: E = mc2. The most important energy generating reactions are equivalent to the schematic reaction: 4H ---> He. The 2 protons + 2 neutrons combined in the He nucleus have less equivalent mass than 4 free protons. This is because of the attractive, negative binding energy in the He nucleus. ∑ moc 2 + E binding = 4m p c 2 . The negative binding energy is like a mass deficit. Where did it go? Lost in γ rays and neutrinos. € Nuclear Fission or Fusion? The elemental abundances and the curve of binding energy shows that fission is not viable. Fission goes only if A > 56. (There are some exceptions.) The abundances of elements with A > 50 are very low. (But fission is important in the Earth’s interior. Eb/A (MeV) 8 Fe56 4 40 80 A Nuclear Fusion A typeical nuclear potential function is shown. The outer part is the Coulomb repulsion for charged particles. The inner ‘well’ level is determined by the balance of the strong nuclear force and the Coulomb force (and the Pauli Principle). 1.4 x 10-13 A1/3 Few MeV E ~ 30 MeV R Nuclear Reactions in Stars In a typical main sequence stellar interior, the average thermal energy is, E = (3/2) kT ≈ 1.5 k (107) ≈ 2 x 10-16 J ≈ 1 keV On the other hand, a typical Coulomb barrier energy is, E = (Z1Z2e2)/R ≈ (4.8 x 10-10)2/(10-13) ≈ 2 x 10-13 J ≈ 1 MeV The barrier is a 1000 times the average particle energy! This estimate is for a collision between protons. For other nucleides we have an extra factor of Z1Z2. This tells us that fusing collisions between high Z nuclei are highly unlikely! € For low Z nuclei there are 2 ways out of the dilemma: 1) The particles have a thermal (Maxwellian) velocity distribution. Fast particles in the tail of the Maxwellian can penetrate the ‘barrier’. But there are not many of these, ∞ N∝ ∫e −v 2 /( 2kT ) v>> kT 4 πv 2 dv. 2) Low energy particles can tunnel through the Coulomb barrier. However, the tunneling probability gets very small as mv2/2 gets much less than the barrier Ec - Ec P ∝ 1 2 exp− 2 mv Ec , 2 1 2 mv P(V2) Where the exponential is called the € Gamow factor. V2 Nature compromises between these alternatives. Most reactions have mv2/2 < Ec, but not so much less that the tunneling is highly improbable. These particles are out in the Maxwellian tail, but not too far out. According to Schwarzschild the typical value of the kinetic energy of a fusing particles is E ≈ 20 keV. P(V2) V2 Energy Generating Fusion Reactions By the 1920s Eddington and others realized that ‘sub-atomic energy’ was the stellar energy source. By the mid-1930s enough nuclear collision data and theory were available to figure out the specific reactions. Strong Coulomb barriers forbid high Z reactions, leaving 2 general types of solution. 1) Direct combination of protons in series up to He4, the next very stable nucleus. 2) An indirect approach, proton bombardment of a heavier catalyst, culminating in an α particle emission. Both approaches are right! • = p-p chain C.F. von Weizsacker 1937, Bethe & Critchfield 1938. • = the carbon cycle Bethe 1939. The Proton-Proton Chain(s) H1 + H1 –––> D2 + e+ + ν +1.44 MeV (14 x 109 yr) D2 + H1 –––> He3 + γ +5.49 MeV (6 sec) He3 + He3 –––> He4 + H1 + H1 +12.85 MeV (106 yr) _______________________________ Epp = 2 (1.44) + 2 (5.49) + 12.85 - 2Eν ≈ 26.7 - 2Eν ≈ 26.2 MeV Why does the 1st reaction take so long? 1) Must form a compound nucleus He2, in which spins of the 2 protons must be anti-parallel. 2) During its brief existence He2 must β-decay. The reactions: He2 ––> 2H1, or He2 + H1 ––> 3H1 are more likely. Why is the 3rd step slower than the 2nd? 1) The Coulomb barrier is 4X higher. 2) It’s hard to find 2He3. There are 2 alternatives to the 3rd step of the p-p chain…. p’ He3 + He4 –––> Be7 + γ +1.59 MeV Be7 + e- –––> Li7 + ν 0.06 Li7 + H1 –––> He4 + He4 17.35 Ep’ = 25.67 - Eν1 - Eν2 ≈ 24.6 MeV p” He3 + He4 –––> Be7 + γ +1.59 MeV Be7 + H1 –––> B8 + γ 0.13 B8 –––> Be8 + e+ + ν 10.78 Be8 –––> He4 + He4 0.09 Ep” = 19.2 - Eν1 - Eν2 ≈ 13.7 MeV _________________________________ p’ and p” probably don’t compete significantly with the main p-p chain in the Sun. However, they provide detectable high energy neutrinos. The Carbon (CNO) Cycle C12 + H1 –––> N13 + γ 1.95 MeV (1.3 x 107 yr) N13 –––> C13 + e+ + ν 2.22 (7 min.) C13 + H1 –––> N14 + γ 7.54 (2.7 x 106 yr) N14 + H1 –––> O15 + γ 7.35 (3.2 x 108 yr) O15 –––> N15 + e+ + ν 2.71 (82 s) N15 + H1 –––> C12 + He4 4.96 (1.1 x 105 yr) ________________________________ ECNO = 26.7 - Eν1 - Eν2 ≈ 25.0 MeV Note that the CNO reaction without the catalysts is essentially: 4H1 –––> He4 + 2e+ + 2ν. The CNO cycle dominates the p-p chain for energy production in stars more massive than the Sun. Diagram of alternative CNO pathways omitted here. Beyond H Burning Helium burning: after burning most of the hydrogen out of their cores most stars initiate He burning via the triple alpha reaction. 4He + 4He → 8Be + γ 8Be + 4He → 12C + γ. However, this is a bit tricky because 8Be has a decay half-life of only 7 x 10-17 sec! Beyond helium burning: After exhausting the helium in their cores massive stars (only) can initiate a wide variety of nuclear reactions. These: 1) provide new energy sources and prolong the star's life, and 2) accomplish nucleosynthesis of all the elements heavier than helium. Among the most important of these advanced burning processes are the alpha reactions, e.g., + 4He → 16O + γ 16O + 4He → 20Ne + γ 20Ne + 4He → 24Mg + γ 24Mg + 4He → 28Si + γ 28Si + 4He → 32S + γ ....... → 56Fe + γ 12C Although there are many side branches. For example, carbon-burning, 12C + 12C → 20Ne + 4He or → 23Na + p or → 23Mg + n (unstable).