Initials: _______
advertisement

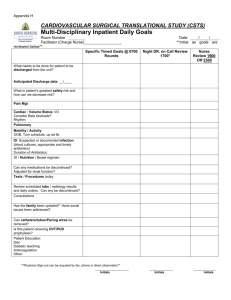
Initials: _______ Chemistry 634, Fall 2014 Midterm 1 • • • • • Closed note, closed book. No calculators allowed. You may use a molecular model set. Answers must be written in the spaces or boxes provided. Other markings will not be considered. Please initial each page at the top. Points will only be awarded for clearly communicated answers. There are 9 total pages to this exam. The last 2 pages were intentionally left blank and may be used for scratch paper. Please be sure your copy has 9 pages before you begin. Name: __________________________________________________ Problem Points 1 _____/30 2 _____/40 3 _____/30 4 _____/20 5 _____/40 6 _____/40 TOTAL _____/200 1 Initials: _______ 1. (30 points) Please provide the reagents needed for each of the following transformations. In some cases, more than one step may be required. OH HO OH HO O O O BnO O O H H Si(i-Pr)3 H Si(i-Pr)3 H Br OMEM OMEM 2 Initials: _______ 2. (40 points) Please fill in the missing intermediates in the following synthesis. HO Me Me BnO CO2Me OTBS (i-Pr)O2CN NCO2(i-Pr) PPh3, MeCO2H OH H HO TIPSOTf 2,6-lutidine K2CO3, MeOH Me Me BnO CO2Me OTBS H OTIPS AcO OAc I OAc O H2, Pd/C O pyridine HO TMS n-BuLi; then ClCO2Me Me Me CO2Me OTBS OTIPS H OCO2Me TMS 3 Initials: _______ 3. (30 points) Please provide formal oxidation state, d-electron count, and valence electron count for the metal in each of the following transition metal complexes. Complex Mes N Ru Formal oxidation state d-electron count Valence electron count N Mes Cl Cl O Me Me Co OC CO Cl Au P(t-Bu)3 H N H N Mn O Cl O N Cl CO Re N Cl CO 4 Initials: _______ 4. (20 points) Please draw a reasonable, arrow-pushing mechanism for the following transformation. Note: This problem is from Grossman’s The Art of Writing Reasonable Organic Reaction Mechanisms. O O N MeN O N Me N O NH Br CO2t-Bu (2 equiv) K2CO3 (2 equiv) N MeN O CO2t-Bu O N Me N N CO2t-Bu 5 Initials: _______ 5. (40 points) A) Please provide a detailed mechanism for the following reaction. You do not need to show the generation of Pd(PPh3)n; you may start your mechanism with Pd(PPh3)n. O O "catalytic Pd(PPh3)n" 10 mol % Pd(OAc)2 40 mol % PPh3 I O NHCO2CH3 O Ag2CO3, THF, 66 °C (73%) O NHCO2CH3 O O O O O B) Please draw a clear transition state model for the C–C bond formation that rationalizes the observed diastereoselectivity. 6 Initials: _______ 6. (40 points) Please propose a synthesis of 2 from aniline (1). In addition to aniline, you may only use reagents containing 2 or fewer carbons as sources of carbon. Me O NH2 1 aniline N Me 2 7 Initials: _______ This page has intentionally been left blank, so that you may use it for scratch paper. 8 Initials: _______ This page has intentionally been left blank, so that you may use it for scratch paper. 9