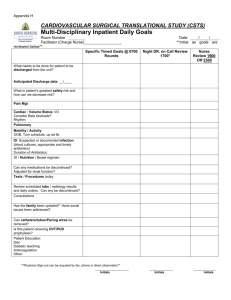
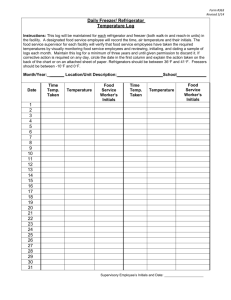
Initials______________ Closed note, closed book. No calculators allowed.
advertisement

Initials______________ Chemistry 634, Fall 2012 Final Exam Prof. Donald Watson 300 points (2 hours) • • • • • Closed note, closed book. No calculators allowed. You may use a molecular model set. Answers should be written in boxes provided. Other markings will not be considered. Scratch paper can be found at the back of the exam packet. Please initial each page at the top. Points will only be awarded for clearly communicated answers. Please make sure you have all pages of the exam packet (12 pages). Name _______________________________________ Question 1: ________/50 Question 2: ________/60 Question 3: ________/40 Question 4: ________/50 Question 5: ________/50 Question 6: ________/50 _____________________ Total:_______/300 Page 1/12 Initials______________ 1) (50 points, 10 points each) Predict the expected major product for each reaction sequence after aqueous work-up, drawing your answer in box provided. Denote stereochemistry where needed. If no reaction is expected, state so. 1) TIPSOTf, 2,6-lutidine 2) BH3, then NaOH/H2O 3) AcO OAc I OAc O OH H H OTBDPS tBuO O AcO O Bu O 2C O H2 (45 psi), 10% Pd/C MeOH CO2tBu N3 AcO OAc 1) LAH 2) NaH, CS2, MeI 3) Bu3SnH, AIBN O BnO Me nBu 2BOTf, iPr 2NEt; Me O O O O Me Me N then H Bn O Cl O N O Cl O O Me Me Cl O O O Me O Me N Zn, NH4OAc S Me Me Me _______/50 points Page 2/12 Initials______________ 2) (60 points) A) In the spaces below, provide the missing reagents and intermediates needed to complete the synthesis. Note: More than one step or reagent may be required over each arrow. 5 pt 5 pt Cl ClMg Me Me O 5 pt Me Me Me OTMS LDA, THF, -78 °C Me OTMS A then O H Cl B O OH C 5 pt TBAF, THF Me OTMS D Cl 5 pt Page 3/12 Initials______________ B) (5 points) In the above scheme, the formation of intermediate B involves the use of “LDA”. Please provide the structure of LDA below. C) (10 points) Considering the scheme above, in the space below, please provide a model that accounts for the formation of intermediate B. D) (5 points) Is the structure of intermediate B best described by a kinetic or thermodynamic model? Please explain using the space below. E) (10 points) In the space below, please provide a model that accounts for the stereochemistry observed in the formation of compound D. ______/60 points Page 4/12 Initials______________ 3) (40 Points) A) In the spaces below, provide the missing reagents and intermediates needed to complete the synthesis. Note: More than one step or reagent may be required over each arrow. Me Me TMSO OH 10 pt O H OTIPS don't worry about stereochemistry for this step Me 1) NaBH4, MeOH, –78 °C 2) TBSCl, imid OH TBSO H OTIPS OTIPS E Me F O LiB(sBu)3H, THF 10 pt TBSO > 95:5 dr H Me TBSO OH H OTIPS G H > 95:5 dr OTIPS H B) (10 Points) In the space below, please rationalize the stereochemistry observed in the conversion of E to F. Recall only clearly communicated answers will receive credit. C) (10 Points) In the space below, please rationalize the stereochemistry observed in the conversion of G to H. Recall only clearly communicated answers will receive credit. ______/40 points Page 5/12 Initials______________ 4) (50 points, 5 points each) Please provide the homolytic bond strengths (BDE’s) for the indicated bonds. Only answers within 3 kcal/mol of the actual value will be given credit. Bond BDE (kcal/mol) Me-H iPr-H tBu-H RO-H CH2=CH-H HOCH2-H MeC(O)CH2-H Et-Cl C≡C (triple) C=O (double) ______/50 points Page 6/12 Initials______________ 5) (50 points) A) (30 points) Please provide a complete and detailed mechanism for the conversion of I to J in the space below. Me Me OH O Me N Me O I 1) DIBAL-H 2) OEt EtO OEt , NaH P O O Me OEt Me OH J O Page 7/12 Initials______________ B) (20 points) In the space below, please provide a model that explains the alkene geometry of product J. ______/50 points Page 8/12 Initials______________ 6) (50 points) In the space below, propose a synthesis of K using 1,3-cyclohexadione and any other reagents that you require. O Me Me O Me K ______/50 points Page 9/12 Initials______________ This page provided for scratch paper. No answers on this paper will be graded. Page 10/12 Initials______________ This page provided for scratch paper. No answers on this paper will be graded. Page 11/12 Initials______________ This page provided for scratch paper. No answers on this paper will be graded. Page 12/12