CHEM 332. Midterm 2
advertisement

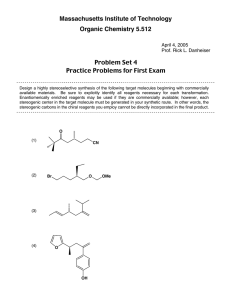
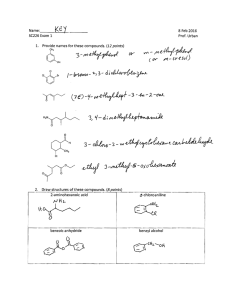
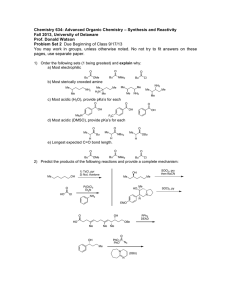
Name: _________________________________ CHEM 332. Midterm 2 Sample Exam Problems Prof Donald Watson The following questions will be representative of the type that will appear on Midterm 2. The actual exam contain approximately 4-6 such questions. 1 Name: _________________________________ 2. (10 points) (a) In acetone, what is the hybridization of the carbonyl C? O H3C CH3 (b) Draw the major resonance structures of acetone. (c) In a carbonyl fragment (C=O), which atom is electrophilic and which is nucleophilic? (d) On the partial structures below, draw a 3-D picture of the C=O π and π* molecular orbitals of acetone. Clearly indicate the geometry, phasing and relative sizes of the lobes. Me Me O Me O Me π π* 2 Name: _________________________________ 3. (20 points) Please provide the missing reagent(s) or expected major product in the empty boxes below. If no reaction is expected, write “No Reaction.” OH Me (a) Me Me Me O Me O O OH (b) O MeO OMe (c) F NO2 NaOCH3 (d) CH3OH, Δ F (e) OH Na2Cr2O7, H2SO4, H2O 3 Name: _________________________________ 4. (8 points) Please draw a reasonable arrow-pushing mechanism for the following reaction. O H H N + O 2N NH2 NO2 catalytic H+ N H N H NO2 NO2 4 Name: _________________________________ 5. (8 points) Provide a synthesis of 1-bromo-1-bromomethylcyclohexane from cyclohexanone. You may use any other reagents you require. Br O ? Br 5 Name: _________________________________ 6. (8 points) Using 1-propanol as your only source of carbons, provide a synthesis of the 3-hexanone. You may use any inorganic reagents. H3C OH ? O H3C CH3 6 Name: _________________________________ 7. (10 Points) For each of the follow reactions, please predict the equilibrium position by indicating if the reaction is strongly endothermic, strongly exothermic or approximately thermoneutral. Write “endothermic”, “exothermic” or “neutral” in the boxes. O O OH + Et3N H2O O Li + + LiOH O OMe OMe O Me + N H Me O Me Me Me H N Me Me Me iPr Me + Me Me + N Li iPr O Me OK Et3NH + O Li Me + N Li O Me Me iPr Me Li N Me Bu Li Me N H iPr Me + Me Me + OH Bu H Me 7 Name: _________________________________ 8. (24 Points) Please provide the necessary reagents to complete the following transformations. O O (a) Me Me Me Me O (b) Me Me Me OH Me OH Bu Bu Me OMe Me O OH (c) O Me (d) Me Me Me Me OH O Me O (e) (f) Me Me Me Br Me Me O Me NH2 8 Name: _________________________________ 9. (18 Points) Please predict the product of the following reactions. If no reaction is expected, state “No Reaction”. Br 1) Mg 2) CO2 3) MeOH, HCl, heat (a) O HCl, heat OH (b) OH 1) LiAlH4 (excess) 2) H3O+ O (c) (d) (e) (f) O DCC, Me2NH O Me OH NaH; then MeI O Me OH LDA, –78 °C; then MeI O Me Et Me 9 Name: _________________________________ 10. (8 Points) Please provide a reasonable arrow-pushing mechanism for the following reaction. O Me HCl, H2O O Me O Me OH 10 Name: _________________________________ 11. (7 points) Using 1-propanol as your only source of carbons, provide a synthesis of propyl 2-methylpentanoate. You may use any inorganic reagents. O Me OH Me Me O Me 11 Name: _________________________________ 12. (7 Points) Please suggest a synthesis of amine 2 starting from acid 1 and any other reagents you need that contain two (2) or less carbons. Me OH Me O 1 Me Me N 2 Me Me 12