Targeted Bioresponsive Nanoparticles Francis C. Szoka, Ph.D. University of California
advertisement

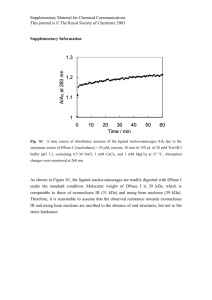
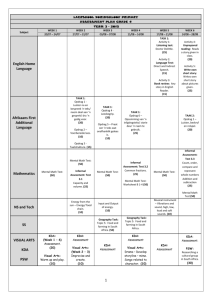
Targeted Bioresponsive Nanoparticles Francis C. Szoka, Ph.D. University of California 2006 Particles Meeting Orlando, Fl May 14, 2006 Happy Mothers’s Day Is there’s plenty of room at the bottom? 10µm Red blood cells 1µm Dendrimers Liposomes Magnetic nanoparticles 100nm 10nm 1nm Aspirin Quantum dots Nature Biotechnology 21, 1161 - 65 (2003); Science 294, 1901 - 3 (2001); Ian Wark Research Institute Summary • Liposomes & Doxil™ • Non-biodegradable dendrimers • Biodegradable dendrimer Lipid Structure Hydrophilic Interface Hydrophobic Time Line for Liposome Drug Carriers • • • • • • • Biomembrane 1965 Enzyme encapsulation 1971 Pharmacokinetics mid 70’s Therapeutics late 70’s Clinical trials 80’s Initial approval early 90’s Doxil™ approval 96 Drug Concentrations at a Tumor Site Following I.V. Administration Free drug [C] Carrierassociated drug Free drug released from carrier accumulated at tumor site Time Structure of Doxil Doxorubicin 85-100 nm Lipid Membrane (Phospholipid + Cholesterol) Polyethylene Glycol F.Martin Doxil Morphology 100 nm F.Martin Plasma concentration (ng/mL) DOXIL Pharmacokinetics 105 T½ = 2.5 d 4 10 103 102 T½ = 3.8 d 101 100 Metabolite appears in few days 10-1 0 F.Martin 5 10 15 20 Time After Infusion (Days) 25 30 Serial Gamma Scintigrams of KS Patient after Pegylated Liposomes Containing 111In-DTPA 4 hrs. F.Martin 24 hrs. 48 hrs. 96 hrs. What Might Further Improve Liposome Drug/Gene Delivery? • Cell targeting – CD44 ligands • Improved cytoplasmic delivery – pH sensitive endosomal escape • Control of surface properties – Redox surface Drug Carriers • Limit drug entry into healthy tissue and cells • Increase drug solubility, modify immunogenicity and enzymatic degradation • Passively target drug to tumors via “leaky” vasculature (EPR effect) - Tumor accumulation enhanced as size increases to 500 nm Blood capillary small diffusible molecules macromolecules Normal cells Lymphatic capillary “Leaky” capillary No lymphatic capillaries Cancer cells “Leaky” capillary Maeda, H., Seymour, L. W., and Miyamoto, Y. Bioconjugate Chem., 1992, 3, 351-362. Dendritic Molecules for Drug Delivery Highly branched • Multiple end-groups for drug attachment • “Surface” functionality can be tailored • Tunable solubility • Decreased flexibility Solubilizing group Drug Branching point Targeting moiety Well-defined, monodisperse < 10 nm • Reproducible synthesis • Reproducible pharmacokinetics Lee et al., Designing Dendrimers for Biological Applications, Nature Biotech. 23,1517, 2005. POLYAMINES USED TO FORM DNA POLYPLEXES H2N NH NH2 NH 2 NH2 NH2 NH2 O N H O N H O NH2 H O N N H O H O N NH2 N H O NH 2 H O N H2N O NH H N O N N H O NH 2 POL N N H N O O N H N HN O PAM O N O NH H N Non-buffering Poor gene transfer O N HN O H O N HN NH2 O NH NH2 HN NH2 Strongly buffering Good gene transfer NH ‘PROTON SPONGE’ HYPOTHESIS H+ H+ B- B BB- pH Cl- Low buffering DNA polyplex pH BB- BB- B- Cl High buffering DNA polyplex STRATEGY TO MEASURE ORGANELLAR CHLORIDE targeting ligand reference Cl- sensor Requirements of Cl- sensor: bright, long-wavelength, ratioable Cl- sensitive (0-100 mM), pH insensitive stable, non-toxic... Sonewame, Szoka & Verkman, 2003 CALIBRATION OF A DEXTRAN CHLORIDE SENSOR BAC-dextran-TMR BAC 10 solution 8 Fred Fgreen 6 cells 4 TMR 2 0 20 40 60 [Cl-] (mM) 80 100 Cl FLUORESCENT SENSING DNA-POLYAMINE POLYPLEX DNA BAC BAC BAC dextran BAC BAC dextran-BAC H2N S S H2N TMR polyamine NH2 TMR polyamine-TMR ENDOCYTOSIS OF DNA POLYPLEX CHLORIDE SENSOR 0-1 min 15 min 30 min 60 min 75 min BAC-dextran-TMR-polyamidoamine / DNA ACIDIFICATION & Cl- ACCUMULATION DEPENDS ON POLYAMINE BUFFERING 7.5 pH 120 [Cl-] (mM) PAM 7.0 PAM 6.5 90 6.0 PEI 5.5 PEI POL 60 POL 5.0 30 0 15 30 45 60 75 0 15 30 45 60 75 time (min) time (min) TMR-polyamine-CF-DNA BAC-dextranTMR-polyamine-DNA LYSIS/PRE-LYSIS IN PEI BUT NOT POL ENDOSOMES PEI 7.5 POL disappeared 7.0 pH 6.5 6.0 5.5 5.0 0 15 30 45 60 75 0 15 time (min) 30 45 60 75 ENDOSOME SWELLING DEPENDS ON POLYAMINE BUFFERING endosome volume (μm3) 3.5 PAM POL / chloroquine 2.5 1.5 POL 0.5 15 30 45 60 time (min) 75 ‘PROTON SPONGE’ MECHANISM H+ H+ B- B BB- pH Cl- Low buffering DNA polyplex pH BB- BB- B- Cl High buffering DNA polyplex A Spectrum of Dendritic Architectures dendrimer-star polymer hybrid “bow-tie” polymer dendronized hyperbranched polymer dendronized linear polymer Polyester Dendrimers: ( )n ( )n ( i )n O O Ph O O ( )n i ii OH ( ii i Ph O O O O O O O O OH OH O O O O O O O Ph O O Ph i ii )n OH O O OH O O O O HO O O O O O O O O O O O O O O O Ph Ph OH O O O O O O O O O OH O O O O O O O Ph ii HO HO O O O O O Reagents: i) O DMAP O O Ph O O O O ( HO )n O O O O OH OH OH OH OH OH OH OH O Ph ii) H2, Pd/C Divergent synthesis with facile purification, high yields Æ Resulting dendrons are degradable, non-toxic Dendritic Molecules for Drug Delivery ( ( ( ( ( ( •32 hydroxyls •3790 Da •Non-toxic •t1/2 < 10 min • 21,000 Da • PDI = 1.02 • Non-toxic • t1/2 = 72 min • Liver accumulation • Poor solubility of drug conjugate at high loadings Padilla de Jesús and coworkers. Bioconjugate Chemistry 2002, 13, 453-461. Dendritic “Bow-Tie” Polymers Poly(ethylene oxide) (PEO) • varying molecular weights • sterically protects payload Dendritic Scaffold • biodegradable polyester • dendron generation varies degree of branching, drug loading potential Gillies and Fréchet J. Am. Chem. Soc. 2002, 124, 14137. Blood Circulation of “Bow-Tie” Polymers Blood Pharmacokinetics in CD-1 Mice (~40 mg/kg) (h) [G-1]–10K (22 kDa) 8±1 33 (24 h) [G-1]–20K (44 kDa) 1.4 ± 0.4 20 (24 h) [G-2]–5K (23 kDa) 11 ± 3 22 (24 h) [G-2]–10K (43 kDa) 26 ± 6 18 [G-2]–20K (87 kDa) 25 ± 8 10 (24 h) [G-3]–5K (45 kDa) 31 ± 2 2 [G-3]–10K (85 kDa) 40 ± 4 2 [G-3]–20K (160 kDa) 50 ± 10 4 [G-1] - 10 K 60 [G-1] - 20 K [G-2] - 5 K 50 [G-2] - 10 K [G-2] - 20 K % Dose / g blood t1/2β % Dose in Urine (48 h) Polymer & MW 40 [G-3] - 5 K [G-3] - 10 K 30 [G-3] - 20 K 20 10 0 0 10 20 30 40 50 Time (h) Summary • [G-3] polymers circulate longer, excreted slower than [G-2] polymers • [G-1] polymers are rapidly cleared with most of the material in the urine and feces after 48 hours. Gilles et al. Mol. Pharm.2005 Similar Mass, Different Elimination rates [G-3]–5K (45 kDa) 31 ± 2 [G-1]–20K (44 kDa) 1.4 ± 0.4 Pores Restrict Reptation of Star Polymers Brochard-Wyart & de Gennes, 1996 Doxorubicin (Dox) O • Anthracycline antibiotic OH COCH2OH OH CH3O O OH H3C O NH2 OH O • Dox has a wellestablished position in the treatment of a variety of human malignancies. • Dox cardiotoxicity is a major clinical handicap limiting its cumulative dosage. Drug Concentrations at a Tumor Site Following I.V. Administration Covalent Attachment of Doxorubicin Synthesis Dendrimer Doxorubicin Tumor Accumulation of “Bow Tie” Polymers Biodistribution of G3-5K at 24 and 48 h 30 24 Hours 48 Hours 20 15 10 5 ss s ce ar ca C Fe rin e U ea rt Lu ng s Li ve St r om ac h Sp In leen te st in es K id ne ys M us cl e Tu m or H d 0 lo o % Dose/g Tissue 25 B • Biodistribution of doxorubicin conjugate of G3-5K (40 kDa) performed in Balb/c mice with subcutaneous C-26 tumors • Tumor/muscle ratio of 16 at 48 h demonstrates enhanced permeability of tumor vasculature Therapeutic efficacy of various control formulations in C-26 colon carcinoma in Balb/c mice. Treatment Number Number Mean P animals survivors survival value day __ PBS 7 0 28 Acetone linked Hydrazone Bowtie 238 mg/kg polymer 8 0 34 0.051 DOX carbamate linked Bowtie 20 mg/kg DOX 10 0 30 0.019 C26 Tumor Progression • • • • • • PBS Doxorubicin 6 mg/kg Bow-tie 1 mg/kg Bow-tie 3 mg/kg Bow-tie 6 mg/kg Bow-tie 20 mg/kg Effect of Bow-tie Dox or Doxil™ on Survival Survival 10 9 8 7 6 5 4 3 2 1 0 Bow-Tie 20 mg/kg 6 mg/kg 3 mg/kg 1 mg/kg 0 10 20 30 40 50 60 Days Survival # of Mice Doxil™ Doxil 20 mg/kg 10 9 8 7 6 5 4 3 2 1 0 Doxil 6 mg/kg 0 10 20 30 Days 40 50 60 Poly-Old Linkage 20 mg/kg Poly-Old Linkage 6 mg/kg Free Dox 6mg/kg Current Status • Liposomes-mature technology – Excellent payload – Biocompatible – Large diameter – Not suitable for all drugs • Dendritric polymers-developing technology – Small diameter – Biocompatible – Suitable for most drugs – Modest payload through chemical linkage Si il l t f i d ith Nanoparticle delivery to the brain Nanoparticles feature: • Targeting • Shielding • Sensing • Reporting Pump Lipid or polymer Convection Enhanced Delivery . Red Blood Cell: 7 μm • Convective flow increases distribution to large volumes • Direct tissue targeting Nanoparticle: 50-100 nm G. Huynh – Szoka group q y Surface Charge Modification by Disulfide Exchange and Ligand Insertion HS-R’ DNA Diacyl lipids Chol-SSR (CTL) PEG-lipid Ligand DNA DNA Chol-SSR’ TAT-PEGlipid R, positive; R’, negative or neutral TATpeptide-targeted NLP for Brain Delivery DNA PEG-lipid Chol-SSR (CTL) TAT-PEG-lipid DOPE TAT-NLP Delivery to U87-MG Brain Tumor No TAT ligand NLP GFP Merge High intensity Low intensity 0.5 cm With TAT ligand 1mm (arrows: tumor border) Intracellular drug transport + + mi + c ro tub ule + + + + + nucleus + + + Biomolecular Adaptors for Retrograde Transport (BART) + + cell + + + + + nucleus membrane Endogenous dynein Novel Cargo BART peptide + ule b u t o micr Rich Cohen- Szoka group Summary • Self-assembled systems make good drug carriers • Improvements needed in targeting, cytoplasmic delivery and surface properties • Sequential assembly, coupled with local delivery, can improve gene transfer to defined tissues Acknowledgements UCSF UCB • Professor Jean • F. Szoka Frechet,Ph.D. • J. MacKay, Ph.D. • Omayra Padilla, Ph.D. • E. Dy Funded by NIH EB3008, CA107268 • Beth Gillies, Ph.D. • Cameron Lee Acknowledgements • • • • • • • • • Szoka group J. MacKay X. Guo W. Li Z. Huang R. Eliaz L. Gagne C. Redemann E. Dy Many past members • D. Deen, Ph.D. • Funded by NIH EB3008, CA107268 The Sequus employees