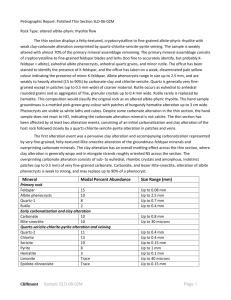
Sericitic and Advanced Argillic Mineral Assemblages and Their Relationship
advertisement

Sericitic and Advanced Argillic Mineral Assemblages and Their Relationship to Copper Mineralization, Resolution Porphyry Cu-(Mo) Deposit, Superior District, Pinal County, Arizona by Alexander Raine Winant A Prepublication Manuscript Submitted to the Faculty of the DEPARTMENT OF GEOSCIENCES In Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE In the Graduate College THE UNIVERSITY OF ARIZONA 2010 1 STATEMENT BY THE AUTHOR This manuscript, prepared for publication in Economic Geology, has been submitted in partial fulfillment of requirements for the Master of Science degree at The University of Arizona and is deposited in the Antevs Reading Room to be made available to borrowers, as are copies of regular theses and dissertations. Brief quotations from this manuscript are allowable without special permission, provided that accurate acknowledgment of the source is made. Requests for permission for extended quotation from or reproduction of this manuscript in whole or in part may be granted by the Department of Geosciences when the proposed use of the material is in the interests of scholarship. In all other instances, however, permission must be obtained from the author. __________________________________________ (author’s signature) _____________ (date) APPROVAL BY RESEARCH COMMITTEE As members of the Research Committee, we recommend that this prepublication manuscript be accepted as fulfilling the research requirement for the degree of Master of Science. Dr. Eric Seedorff__________________________ Major Advisor (type name) (signature) _____________ (date) Dr. Mark D. Barton____________________________ (type name) (signature) _____________ (date) Dr. Frank K. Mazdab _________________________ (type name) (signature) _____________ (date) 2 Sericitic and Advanced Argillic Mineral Assemblages and Their Relationship to Copper Mineralization, Resolution Porphyry Cu-(Mo) Deposit, Superior District, Pinal County, Arizona Alexander R. Winant and Eric Seedorff Lowell Institute for Mineral Resources, Department of Geosciences, University of Arizona 1040 East Fourth Street, Tucson, Arizona 85721-0077 Hamish R. Martin Resolution Copper Company, 47206 N. Magma Shaft #9 Road, Superior, Arizona 85273 Frank K. Mazdab and Mark D. Barton Lowell Institute for Mineral Resources, Department of Geosciences, University of Arizona 1040 East Fourth Street, Tucson, Arizona 85721-0077 3 Abstract The Resolution deposit is a giant, deep, high-grade deposit in the Laramide porphyry copper province of Arizona that is currently being developed. This study focuses on the features at Resolution that formed from acidic hydrothermal fluids (including sericitic and advanced argillic alteration types) that are well developed in the upper part of the system. The distribution of alteration-mineralization features are illustrated along two, roughly perpendicular fences of drill holes that were logged with concurrent mineral identifications made with a PIMA™ infrared spectrometer and ultraviolet light and supplemented with subsequent reflected and transmitted light petrographic observations. Hydrothermal minerals formed during intense hydrolytic alteration at Resolution commonly are related to multiple superimposed, crosscutting events. Though showing some degree of stratigraphic control, particularly at deep levels, the distribution of hydrothermal minerals and mineral assemblages shows only weak degrees of structural control at the deposit scale. The intermediate sulfidation opaque assemblages containing chalcopyrite characterize the many hydrothermal mineral assemblages that formed potassic alteration of igneous rocks, skarn, and calc-silicate hornfels, which are best developed outside the region of this study. Earlier sericitically altered rocks contain pyrite ± chalcopyrite, but later sericitic and advanced argillic assemblages contain higher sulfidation state opaque assemblages, such as pyrite + bornite ± chalcocite with kaolinite, dickite, and topaz, with lesser alunite, pyrophyllite, and zunyite. 4 Veins with assemblages characteristic of advanced argillic alteration consistently offset veins associated with sericitic alteration. Most of the advanced argillic assemblages at Resolution formed at relatively low temperatures, stable with kaolinite and dickite. Resolution contains fairly high levels of fluorine. The most important fluorine-bearing minerals are biotite (~3-4 wt% F), topaz (~11-12 wt% F), fluorite (~49 wt% F), and sericite (~1 wt% F), although other fluorine-bearing phases also are locally present (e.g., zunyite, 6-7 wt% F). Topaz formed at Resolution during advanced argillic alteration and the mineral has a relatively fluorine-poor composition (XF-tpz ~0.6), as is topaz from other base-metal lode deposits such as Butte, in contrast to topaz in those porphyry deposits in which a more fluorine-rich topaz occurs in sericitic and potassic assemblages. Resolution is a relatively arsenic-poor system, in strong contrast to the nearby Magma vein system. The deeper part of the ore body, where potassic alteration dominates, is nearly arsenic-free, whereas the upper part of the copper ore body is arsenic-bearing. Although enargite has been observed petrographically, arsenic occurring in solid solution in other sulfides (e.g., arsenic-bearing pyrite) may be responsible for many of the local spikes in arsenic content at Resolution. Introduction Intense hydrolytic alteration of the sericitic and advanced argillic types, though known also from other types of hydrothermal ore deposits, occurs commonly in three related types of 5 magmatic-hydrothermal ore deposits, porphyry deposits, base-metal lode deposits, and acid-sulfate or high-sulfidation epithermal deposits (Meyer and Hemley, 1967; Hemley et al., 1980; Einaudi, 1982; Arribas, 1995; Seedorff et al., 2005a). Intense hydrolytic alteration, regardless of deposit type, can be pervasive or can be confined to structures or stratigraphic units; rocks exhibiting intense hydrolytic alteration can be barren to highly mineralized. Where mineralized, high- to very-high sulfidation state opaque minerals commonly are associated with advanced argillic alteration of silicate minerals (Meyer and Hemley, 1967; Einaudi, 1982; Einaudi et al., 2003). Intense hydrolytic alteration is characteristic of shallower levels of certain porphyry systems (e.g., Red Mountain, Arizona; Resolution, Arizona; El Salvador, Chile; Central deposit, Oyu Tolgoi, Mongolia) and base-metal lode deposits (e.g., Bisbee, Arizona), though sericitic and advanced argillic alteration can persist to deep levels, as at Butte, Montana (Bryant, 1968; Meyer et al., 1968; Corn, 1975; Gustafson and Hunt, 1975; Bodnar and Beane, 1980; Hedenquist and Lowenstern, 1994; Reed and Meyer, 1999; Watanabe and Hedenquist, 2001; Manske and Paul, 2002; Khashgerel et al., 2009). For the high-sulfidation epithermal deposits, links to porphyry systems are well established in certain cases (e.g., Lepanto- Far Southeast in the Philippines) but to date are lacking in many other districts (e.g., Goldfield, Nevada, and Yanacocha, Peru) (Einaudi, 1982; Arribas et al., 1995; Harvey et al., 1999; Sillitoe and Hedenquist, 2003). Likewise, it is not necessarily clear whether fluids that formed intense hydrolytic alteration represent evolution of fluids that produced potassic alteration at earlier stages or whether they 6 represent a temporally distinct hydrothermal system (e.g., Meyer et al., 1968; Brimhall and Ghiorso, 1983). Rocks exhibiting intense hydrolytic alteration commonly represent a special challenge in identifying mineral assemblages, defined as a group of minerals that appear to be stable together at the mesoscopic scale and to have formed contemporaneously (e.g., Seedorff et al., 2005a). In many cases, the hydrothermal minerals clearly are related to multiple superimposed, crosscutting events, yet the identity of the products of each event may be difficult to determine at the hand specimen scale. Moreover, the silicate minerals commonly are light colored, fine-grained, and difficult to identify with the naked eye or hand lens and in some cases petrographically, such as distinguishing between sericite and pyrophyllite. Even where the minerals can be determined by with aid of infrared spectrometers and X-ray diffraction, the textural relationships generally are lost at the spatial scales of such determinations, i.e., the minerals identified may have formed in multiple events, so the nature of the mineral assemblage remains uncertain. For these reasons, the identification of mineral assemblages within areas of intense hydrolytic alteration commonly is avoided or not deemed possible (e.g., Khashgerel et al., 2006), thereby limiting the types of geochemical or genetic conclusions that might be drawn. This study was conducted at the Resolution deposit in Arizona. The study focuses on the upper part of the Resolution system where sericitic and advanced argillic assemblages are prevalent, building on work by Manske and Paul (2002), Ballantyne et al. (2003), Schwarz (2007), and the geologic staff at Resolution, especially on previous work by Troutman (2001) 7 and Harrison (2007) on sericitic and advanced argillic alteration. The purposes of this study are to document the distribution, abundance, and compositions of associated hydrothermal minerals, to attempt to define the mineral assemblages that constitute sericitic and advanced argillic alteration, to determine the lateral and vertical changes in abundance of sericitic and advanced argillic alteration,, and to document the relative ages of associated veins. We show that acidic hydrothermal fluids at Resolution formed a variety of vein types and mineral assemblages, though some uncertainty remains in defining assemblages. Most assemblages at Resolution are of the sericitic and advanced argillic types, but they include some assemblages that are transitional between those two types. Most of the advanced argillic assemblages formed at relatively low temperatures, stable with kaolinite and dickite. The Resolution deposit contains fairly high levels of fluorine (Schwarz, 2007), and we document that fluorine occurs mainly in topaz, sheet silicate minerals, and fluorite and that the onset of topaz deposition occurred during advanced argillic alteration. Resolution is a relatively arsenic-poor system (e.g., Fig. 15 of Manske and Paul, 2002), in strong contrast to the nearby Magma vein system, and we show that local spikes in arsenic content at Resolution probably occur mostly where arsenic occurs as a minor component in other sulfides (e.g., arsenic-bearing pyrite), rather than as occurrences of discrete arsenic minerals, such as tennantite or enargite. After reviewing the geologic setting of the district, this paper will illustrate the distribution of key metals and hydrothermal minerals, document the advanced argillic and sericitic assemblages and their relative ages as documented during core logging of two, 8 approximately orthogonal fences of drill holes, with concurrent use of a PIMA™ infrared spectrometer and ultraviolet light and subsequent reflected and transmitted light petrographic observations to aid mineral identification. Elemental distributions and mineral compositions that were determined by electron microprobe and the scanning electron microscope, especially of arsenic- and fluorine-bearing minerals, further constrain the geochemical environment of hydrothermal fluids. The silicate components of the assemblages are classified into alteration types using activity ratio diagrams, and the sulfide-oxide component of the assemblages are classified by sulfidation state, to assess the degree of correlation between advanced argillic alteration and high-sulfidation state mineral assemblages. The geochemical stabilities of successive mineral assemblages are used to define the possible evolutionary paths of fluids. The results have potential applications to exploration, production planning, milling, and smelting. Geologic Setting The Resolution deposit is located in the Superior (Pioneer) district, Pinal County, Arizona, north of Tucson and east of Phoenix (Fig. 1). Porphyry-related deposits in the Superior district formed within the Late Cretaceous to early Tertiary Laramide arc, which has been variably dismembered and tilted by mid- to late Tertiary normal faulting (Titley, 1982; Wilkins and Heidrick, 1995; Lang and Titley, 1998; Maher, 2008; Seedorff et al., 2008; Stavast et al., 2008). The geology of the Superior district has been mapped and described by Peterson (1969), Hammer and Peterson (1968), and Manske and Paul (2002), and is summarized here. The 9 Proterozoic Pinal Schist forms the local basement of the district and adjacent areas and is overlain by Proterozoic strata of the Apache Group, which consists in ascending order of the Pioneer Formation, the Dripping Spring Quartzite, the Mescal Limestone, and locally by basaltic lava flows. The Apache Group was intruded at 1.1 Ga by a series of diabase sills, which also intrude the underlying Pinal Schist as sheets of similar orientation. The Apache Group is capped by the Proterozoic Troy Quartzite. The Proterozoic strata are overlain disconformably by >800 m of Paleozoic carbonate and clastic rocks that now dip east at 35˚ to 40˚. The Paleozoic stratigraphic section includes the Cambrian Bolsa Quartzite, Devonian Martin Formation, Mississippian Escabrosa Limestone, and Pennsylvanian-Permian Naco Group. Mesozoic sedimentary and intermediate volcanic and volcaniclastic rocks, correlated regionally with quartzites of the Pinkard Formation and with the Williamson Canyon Volcanics, respectively, are preserved inside a down-faulted structural block that includes the Resolution deposit. The Mesozoic, Paleozoic, and Proterozoic rocks are intruded by felsic porphyry dikes and sills, perhaps soon after periods of thrust, normal, and strike-slip faulting (Manske and Paul, 2002). Laramide rocks have proven to be a geochronologic challenge to date accurately, but the Resolution center probably includes rocks formed at ~63 Ma, and other porphyries in the district may be as old as ~69 Ma (Ballantyne et al., 2003; Seedorff et al., 2005b). Pre-Tertiary rocks are unconformably overlain by the Whitetail Conglomerate, an east-dipping growth sequence deposited in a half-graben that constituted the Whitetail sedimentary basin. The basal unconformity and the lowest beds exposed in this sequence dip at 10 least 25° to the east, with strata higher in the sequence dipping less steeply. The 25° dip likely represent the minimum amount of post-ore eastward tilting of the deposit, although the actual amount of tilting is uncertain. The Whitetail Conglomerate is overlain by the Apache Leap Tuff, which is a welded ash-flow tuff dated at 18.6 Ma that can exceed 400 m in thickness and dips 10˚ to 15˚ to the east (Peterson, 1969, 1979; Ferguson et al., 1998; McIntosh and Ferguson, 1998). Until the last decade, the Superior district was known primarily for production from the Magma vein and from related mantos that replace selected beds in the Paleozoic carbonate sequence (Short et al., 1943; Gustafson, 1961; Hammer and Peterson, 1968; Paul and Knight, 1995; Friehauf, 1998; Pareja, 1998), and the Magma vein and mantos have similarities to other base-metal lode deposits, such as the Butte, Montana, and Cerro de Pasco, Peru (Einaudi, 1982). The Magma vein and mantos occur north of the town of Superior and extend eastward under the Apache Leap Tuff toward the #9 Shaft. The Resolution deposit occurs beneath the Apache Leap Tuff, largely south and east of the Magma vein (Manske and Paul, 2002; Ballantyne et al., 2003; Schwarz, 2007; Fig. 1). Manske and Paul (2002), among others, have argued that the Magma vein and Resolution deposit are distinct magmatic-hydrothermal systems, but the relationship between the two centers, Magma and Resolution, remains controversial. The Resolution Deposit The Resolution deposit is a major porphyry copper deposit first discovered in the mid-1990s (Manske and Paul, 2002; Paul and Manske, 2005). The known extent of the deposit 11 and its development toward becoming a mine have been enhanced more recently by Resolution Copper Mining LLC (Ballantyne et al, 2003; Schwarz, 2007; Anonymous, 2010), a joint venture between Resolution Copper Company (55%), a subsidiary of Rio Tinto plc, and BHP Copper, Inc. (45%), a subsidiary of BHP Billiton Ltd. The top of the ore body is ~1.5 km below the surface, and Resolution Copper Mining LLC plans to use a panel cave method to mine the deposit beginning in the year 2020. At this time, the deposit is known only from drill core obtained from holes that are primarily greater than 2 km in length, steeply plunging, and irregularly spaced (e.g., Anonymous, 2008). Resolution Copper Mining LLC reported in March 2010 that the deposit has an Inferred Mineral Resource of 1.624 billion tonnes at a grade of 1.47 per cent copper and 0.037 per cent molybdenum (Anonymous, 2010). The Resolution deposit is geologically distinctive for several reasons (Manske and Paul, 2002; Schwarz, 2007), including: (1) High hypogene copper grades occur in a variety of environments, principally as chalcopyrite in diabase, calc-silicate rocks, and intermediate volcanic rocks, which tend to occur at relatively deep (pre-tilt) levels, but also as bornite ± digenite in rocks that tend to occur at shallower levels. (2) Large volumes of rock are affected by moderate to intense hydrolytic alteration of the sericitic and advanced argillic types. (3) Bornite, rather than chalcopyrite, is abundant in intense sericitic alteration. (4) Enargite is rare to absent in advanced argillic alteration, as is tennantite in sericitic alteration, in marked contrast to the nearby Magma vein. 12 Methods At the project site, the senior author relogged >2,500 m of core from eight drill holes to supplement existing drill logs and multi-element assays, with a focus on attempting to identify the hydrothermal mineral assemblages (i.e., silicate, sulfides, and other minerals) in vein fillings and alteration envelopes and the crosscutting relationships between partially superimposed events. The holes selected for logging are oriented along two crossing sections, with data projected onto sections oriented at azimuths of approximately 100 and 180 (Fig. 1), which are referred to as nominally east-west and north-south sections, respectively. Geologic logging included using a hand lens to observe and record sulfide and silicate volume percent estimates, to estimate abundances of alteration minerals, and to measure veins (angle to core axis, abundances, widths). Data simultaneously were collected with the PIMA™ (Portable Infrared Mineral Analyzer) short-wave infrared spectrometer during every logging interval, and samples of drill core frequently also were observed under ultraviolet (UV) illumination to check for the presence of hydrothermal topaz, which strongly fluoresces blue-white under short-wave UV (Marsh, 2002). Although the PIMA™ spectrometer occasionally gives spurious information (e.g., indicating presence of stilbite where none is present), previous work by Troutman (2001) at Resolution that linked visual core logging and PIMA™ spectrometer analysis in the field with petrography and X-ray diffraction analysis in the laboratory demonstrated the overall utility of using the PIMA™ spectrometer to aid in identifying the fine-grained minerals that are typical of sericitic and advanced argillic alteration 13 at this deposit. In this study, 355 samples were analyzed using the PIMA™ and short-wave UV. The various geologic and geochemical observations and measurements subsequently were plotted on the two cross sections using Microsoft Excel, using color-coded data points to portray intensity or grade, with the purpose of examining spatial distributions and correlations. The most instructive features were imported into a drafting program, where they were manually contoured, as described further in a later section. In laboratories at the University of Arizona, transmitted and reflected light petrography was carried out on 70 polished thin sections, and these data were added to the cross sections and incorporated into tables. A Scanning Electron Microscope (SEM) and an electron microprobe were used to confirm the identities of minerals, as well as to confirm presence of and/or to quantity the abundance of certain elements, such as arsenic and fluorine. A CAMECA SX 50 electron microprobe was used to obtain quantitative compositions of biotite, sericite, topaz, and clay minerals using routine methods and standards. Distribution of Rock Types, Alteration, and Selected Elements Geologic cross sections and assays from the Resolution project provide the geologic and geochemical framework of the deposit and a basis for interpreting data collected in this study. The distribution of rock types and alteration zones are taken directly from the block model that was developed by Resolution geologists, which is based on drill holes throughout the deposit, rectified in three dimensions. The understanding of the geology continues to be improved by 14 continued study and analysis and by drilling additional holes (e.g., Anonymous, 2008, 2010), The present understanding of the distribution of rock types within the deposit generally follows that of Ballantyne et al. (2003), but the structural history of the district remains uncertain. There are both pre-ore and post-ore faults in the district with large displacement, but the faults present within the cross sections have relatively modest offsets and are thought to be largely pre-ore in age. Multi-element geochemical data from drill holes in the two selected cross sections were contoured by hand, attempting to be consistent with existing geologic observations (e.g., degree of lithologic control of mineralization). Nonetheless, these contours are non-unique interpretations and are subject to considerable uncertainty, given the current spacing and orientation of drill holes. Two cross sections are shown here (Fig. 1). The deposit is elongate in an east-west direction, and the east-west cross section illustrates at least some of the effects of eastward, post-ore tilting of the deposit. Additional holes drilled farther east and to greater depths may be required to describe the full geometry and size of the system. The north-south section, in contrast, is a slice through the system that largely obscures the effects of tilting by movement on normal faults and seemingly displays the full north-south extent of the Resolution system. Distribution of rock types Figure 2 shows the distribution of rock types at Resolution in both cross sections. Beginning from the bottom of the cross sections, the first ~ 600 m consists mostly of schist 15 overlain by stratified sedimentary rocks, consisting mostly of Proterozoic Pinal Schist, quartzite, and limestone (mostly converted to skarn), with sills and sheets of diabase, Paleozoic quartzite and limestone, and Cretaceous quartzite (Fig. 2). A 600- to 700-meter thick Cretaceous volcaniclastic unit that dips to the northeast overlies this sequence of units. Two main types of porphyry stocks and dikes intrude the Cretaceous volcaniclastic unit and older rocks, including a porphyry stock on the eastern side of the east-west section (Fig. 2). The pre-Tertiary units, none of which is post-ore in age, are overlain by Tertiary post-ore units, the Whitetail Conglomerate and overlying Apache Leap Tuff (Fig. 2). Distribution of alteration zones The Resolution block model includes a field for the dominant alteration type or zone (Fig. 3). The model describes six alteration zones, the first five of which are hypogene in origin: chlorite ± epidote ± calcite ± adularia (referred to as propylitic), quartz + sericite + pyrite (sericitic), biotite ± K-feldspar ± chlorite ± anhydrite (potassic), quartz + sericite + pyrite overprinting biotite ± K-feldspar (sericitic/potassic), dickite ± pyrophyllite ± alunite ± zunyite ± andalusite (advanced argillic), and the supergene hematitic leached cap (Fig. 3). In this simplified view, the alteration in sedimentary rocks is assigned the same type as those of nearby igneous rocks. The base of the cross sections is composed of potassically altered rocks or potassically altered rocks with a sericitic overprint (Fig. 3); these are confined mainly to Proterozoic schist, diabase, and lesser Mescal Limestone. Rocks altered to sericitic and advanced argillic alteration 16 occupy a large fraction of the model (Fig. 3), affecting large volumes of porphyry and extending out into Cretaceous volcaniclastic rocks and other lithologies, including quartzites and locally limestones and diabase. Aside from the potassic alteration that dominates the deeper parts of the system, the two types of hydrolytic alteration in the block model generally show a central core of advanced argillic alteration, giving way upward and outward to sericitic alteration (Fig. 3). The Tertiary unconformity clips the top of the core of advanced argillic alteration in certain sections; there, advanced argillic alteration may have extended further upward, perhaps as a pipe or an upward-expanding funnel (see also Fig. 15 of Manske and Paul, 2002). In the north-south section, sericitic alteration in turn is zoned upward and outward in both directions to propylitic alteration. On the east-west section, the zoning pattern is incomplete on the eastern side of the section where sericitic alteration pervades a porphyry stock, because of an absence of drilling further to the east where the Tertiary overburden continues to thicken (Fig. 3). The block model locally shows “fingers” of advanced argillic alteration extending downward directly into potassic alteration, although sericitic alteration also may underlie advanced argillic alteration (Fig. 3). The hematitic leached cap was formed in Cretaceous volcaniclastic rocks and is overlain by the Whitetail Conglomerate. Distributions of copper, arsenic, iron, and sulfur Assays of selected elements in the Resolution database are useful for addressing aspects of the distribution of alteration-mineralization assemblages and possible linkages between silicate and sulfide mineral assemblages (e.g., Meyer and Hemley, 1967). Data are presented here 17 for copper, arsenic, iron, and sulfur. Copper: Copper grades exhibit a strong control by host rock lithology (Anonymous, 2008). The highest copper grades (>3 wt%) flare out from the upper volumes of porphyry into Cretaceous volcaniclastic rocks and in beds of Proterozoic Mescal Limestone and sheets of diabase (Fig. 4). Copper grades of 1 to 3 wt% can occur in most lithologies with the exception of Proterozoic Pinal Schist. The highest copper grades are observed mostly in advanced argillic altered rocks, although there are significant volumes of rock interpreted as sericitic, sericitic/potassic, and potassic alteration in the RCC alteration block model. Cretaceous and Proterozoic quartzites generally have lower copper grades than adjacent rock units. Arsenic: As noted by previous workers (e.g., Fig. 15 of Manske and Paul, 2002; Schwarz, 2007), Resolution is a relatively arsenic-poor system. Troutman (2001), Manske and Paul (2002), and Harrison (2007) did not report observing either enargite or tennantite. These minerals also were not observed in this study, although petrographers for Resolution have identified enargite in thin section. Manske and Paul (2002) note that it is uncommon for arsenic levels to exceed 100 ppm, even in areas with abundant chalcocite/digenite with copper grades >1 percent, and fewer than 2% of the intervals in the present Resolution assay database exceed 300 ppm As. Arsenic assays, nonetheless, do display a systematic spatial distribution. In this study, arsenic assays from drill holes in the two cross sections considered herein are contoured. The upper part of the copper ore body is arsenic-bearing in both cross sections (cf. Fig. 5 and Fig. 4), whereas the deeper part of the ore body, where potassic alteration dominates (Fig. 4), is nearly arsenic-free (Fig. 5). 18 Representative samples from intervals with elevated arsenic levels that were logged in this study subsequently were examined in reflected light and with a Scanning Electron Microscope (SEM) to assess the mineralogic host of arsenic. Neither enargite nor tennantite was detected; rather, rocks with high arsenic levels showed a relatively uniform distribution of the element in other sulfide minerals, such that arsenic-bearing pyrite is probably the source of most local spikes in arsenic abundance [Fig. 6]. Iron: Iron contents can reflect both primary iron contents (e.g., high in diabase and low in quartzite) and hydrothermal modifications, especially by metasomatic addition of iron during hydrothermal alteration-mineralization, mostly as iron and copper-iron sulfide minerals. Nonetheless, the highest contents of iron (Fig. 7) largely coincide with regions of advanced argillic and sericitic alteration, extending downward into the underlying area of potassic alteration (Fig. 3). Iron is enriched, however, on the northern part of the north-south section (Fig. 7B), where advanced argillic alteration is better developed (Fig. 3B). The distribution of iron is even more asymmetric on the east-west section (Fig. 7A, where the highest iron contents also occur within advanced argillic alteration, and the contours of iron abundance either truncate upward and eastward against the tilted Tertiary erosion surface and/or iron contents diminish eastward into a body of relatively silicic porphyry. The highest iron contents (>10wt%) occur almost exclusively in Cretaceous volcaniclastic rocks and Mescal Limestone For comparison, relatively fresh diabase contains ~6-10 wt% Fe, as 8-11 wt% FeO and 1-3 wt% Fe2O3 (e.g., Wrucke, 1989). 19 Sulfur: Sulfur contents almost entirely reflect metasomatic additions of sulfur as sulfate (mostly in hydrothermal anhydrite) or as various sulfide minerals. As in the case of iron, sulfur is most concentrated in the northern and western regions of the cross sections (Fig. 8). Highest sulfur contents (>10 wt% S) are in Cretaceous volcaniclastic rocks, Mescal Limestone, diabase, and porphyry. Sulfur contents of >10 wt% S also correspond mostly to regions of advanced argillically altered rocks and, to a lesser extent, sericitically altered rocks. Key hydrothermal minerals: Distribution and compositions The distribution and abundance of key hydrothermal minerals are presented in Figures 9 to 15, with supporting petrographic observations in Figure 16, which will lead subsequently to an interpretation of the hydrothermal mineral assemblages. Representative mineral compositions from a reconnaissance electron microprobe study are presented in Tables 1 and 2, and complete results are present in an appendix of Winant (2010). The descriptions are grouped as follows: (kaolinitic) clay, sericite, other minerals commonly associated with, though not necessarily characteristic of, advanced argillic alteration in porphyry copper systems (topaz, pyrophyllite, zunyite, alunite, and APS minerals) are followed by other silicate and non-sulfide minerals, and finally various sulfide minerals. The distributions are based principally on visual estimates made during drill logging, but in some cases other types of information also were available, including PIMA™ spectrometer measurements and petrographic observations (e.g., eight types of data were consulted to derive contours of intensity of advanced argillic and sericitic alteration). The data were manually 20 contoured, with qualitative to semi-quantitative contour intervals chosen based on the resolution of the data. Contours are shown with solid lines where interpolated between the senior author’s observations in logged portions of drill holes; dashed lines represent inferred extensions of those contours based on data other than those drill logs. Clay: The term clay here refers only to the aluminum silicates kaolinite, dickite, and halloysite, identified principally with the PIMA™ spectrometer. Although montmorillonite (smectite) is also a clay mineral, it is discussed separately below. Although kaolinite occurs within the leached cap and there is probably all or in part of supergene origin (e.g., Troutman, 2001), most of the kaolin group minerals logged at Resolution occur with pyrite and other sulfides, where the clay minerals are interpreted to be of hypogene origin (Fig. 16D). The occurrence of kaolin group minerals at Resolution includes white kaolinite in strongly silicified zones containing bornite ± chalcocite and massive translucent pale green dickite with pyrite, bornite, and chalcocite (Troutman, 2001; Manske and Paul, 2002). The kaolin group minerals are present mostly in two general areas: (1) extending out from porphyry through Cretaceous volcaniclastic rocks, and (2) stratabound occurrences within certain sedimentary units in the lower part of the cross sections (Figs. 2 and 9). The regions with the most abundant kaolin group minerals coincide with regions of the RCC block model that are assigned to the advanced argillic alteration zone but extending into the sericitic zone, whereas moderate abundances of clay extend downward into the potassic zone and outward into the propylitic zone (Figs. 3 and 9). Microprobe analyses of 11 kaolin group minerals contain ~0.2 to 1.1 wt% F; 21 representative analyses are shown in Table 1. Sericite: PIMA™ measurements identified 170 samples containing muscovite and 104 samples containing illite (out of a total 253 measurements), supported by petrographic observations (Fig. 16D,E). These minerals were grouped together for the purposes of describing sericitically altered rock. High sericite values are observed across the two cross sections (Fig. 10); rocks containing such values include Cretaceous volcaniclastic rocks, Cretaceous quartzites, diabase, Mescal limestone, Pinal Schist, and quartzite (Fig. 2). High sericite values are contoured primarily in areas that the RCC block model assigns to the advanced argillic, sericitic, and sericitic/potassic alteration zones (Fig. 3). The electron microprobe was used to analyze 16 grains of sericite, and representative analyses are reported in Table 1. The compositions vary widely between 2.9 and 9.4 wt% K2O with negligible Na2O, and none of the analyzed grains is a dioctahedral mica. The mean K2O content of 6.2 wt%, which corresponds to a mean occupancy of the A site of only ~60%, implying that the grains have non-muscovite components such as illite (e.g., Bailey, 1984; Brigatti and Guggenheim, 2002). The major-element compositions of sericite from Resolution are thus distinct from those of sericite analyzed from some other porphyry systems, in which the A site generally is almost fully occupied (e.g., Koloula, Guadalcanal, Chivas, 1978; San Manuel-Kalamazoo, Arizona, Guilbert and Schafer, 1979; Santa Rita, New Mexico, Parry et al., 1984; Henderson, Colorado, Seedorff and Einaudi, 2004a). The analyzed sericite grains also contain ~0.5 to 1.7 wt. percent F, which overlaps with but extends to higher levels than observed 22 for clay. Topaz: Topaz occurs widely across certain parts of the deposit (Fig. 11), and suspected occurrences can be easily confirmed with the assistance of short-wave UV light, with the PIMA™ spectrometer, and petrographically (Fig. 16G). As noted by Marsh (2002), topaz occurs most commonly in alteration envelopes on pyrite veins. Topaz is in equilibrium with bornite and chalcocite and has not been observed in equilibrium with chalcopyrite. High and moderate topaz values are observed in Cretaceous volcaniclastic rocks, porphyry, Cretaceous quartzite, and Proterozoic limestone, quartzite and diabase (Figs. 2 and 11). About half of the topaz identified in this study occurs in rocks assigned to the advanced argillic zone in the RCC block model, and the remainder occurs in the sericitic zone (Fig. 3). Six topaz grains from Resolution were analyzed by electron microprobe; representative analyses are shown in Table 2. Topaz is uniformly relatively fluorine-poor, containing 11-12 wt percent F. This is equivalent to a mole fraction of fluor-topaz in topaz solid solution (XF-Topaz) of 0.58 – 0.64. As discussed further in a later section, the fluorine content of topaz helps to constrain the geochemical environment of its formation (e.g., Barton, 1982; Seedorff, 1986; Seedorff and Einaudi, 2004b), and XF-Topaz) of ~0.6 is indicative of forming in an advanced argillic alteration environment. Pyrophyllite and andalusite: In this study, only three occurrences of pyrophyllite, confirmed by PIMA™ spectrometer, were documented on the two cross sections that were studied (Fig. 12). All occurrences of pyrophyllite occur as intergrowths with alunite, chalcocite, 23 and bornite and are present in the same region (Fig. 12) where topaz is present in moderate or high levels (Fig. 11). Three isolated occurrences of pyrophyllite, with alunite, topaz, and kaolinite, also were detected in a large-scale infrared spectral reflectance study commissioned by RCC that used the HyLogger technique in two drill holes (Huntington and Yang, 2009). The relative paucity of pyrophyllite (Fig. 12) compared to clay (Fig. 9) at Resolution that was observed in this study is consistent with the earlier observations (Troutman, 2001; Manske and Paul, 2002; Harrison, 2007). Andalusite has not been documented at Resolution by earlier workers (Troutman, 2001; Manske and Paul, 2002; Harrison, 2007; Schwarz, 2007). Andalusite was not observed in core logging or petrographic examinations made in this study, but several samples submitted by Resolution geologists for petrographic description have reported local occurrences of andalusite. Phases indicative of quartz-undersaturated conditions, such as diaspore and corundum (Hemley et al., 1980), also were not observed. Alunite: High alunite values occur in the Cretaceous volcaniclastic rocks, although there are also rare occurrences in Cretaceous quartzites, porphyry, and Proterozoic Mescal Limestone, diabase, and quartzite (Figs. 2, 12, 16F). High alunite values occur in rocks assigned to both the advanced argillic and sericitic zone of the RCC block model (Figs. 3 and 12), but RCC has observed alunite at deep levels of the deposit (e.g., the bottom of hole RES-2A) in rocks assigned to the zone with sericitic alteration superimposed on potassic alteration. Harrison (2007) observed several instances of alunite nucleating around cores of APS minerals (see below). 24 APS minerals: Although not observed in this study, aluminum-phosphate-sulfate (APS) minerals were identified in felsic protoliths by Harrison (2007) using the scanning electron microscope. Harrison (2007) observed that the minerals occur either with an “irregular, ragged” texture in kaolinite, form cores of alunite, or occur as “dendritic rapidly cooled masses and zoned fragments in alunite vein matrix” that may be pseudomorphs of precursor apatite. Electron microprobe analyses by Harrison (2007) revealed APS compositions intermediate between the end-members hinsdalite, woodhouseite, and svanbergite, with no evidence for the weilerite component, as arsenic occurred below the detection limit. Zunyite: Seven occurrence of zunyite were recorded in this study at the locations plotted on Figure 12, coinciding with areas of the cross sections that contain moderate and high levels of topaz. Zunyite also is reported by Troutman (2001) and Manske and Paul (2002). Two electron microprobe analyses of zunyite, shown in Table 2, contain 6 to 7 wt percent F and more than 2 wt percent Cl. Biotite, chlorite, and fluorite: In this study, core generally was not logged in areas with well-developed potassic or propylitic alteration, where biotite and chlorite are abundant. Nonetheless, several potassically altered specimens were collected, and hydrothermal biotite was analyzed by electron microprobe (Table 1). The analyzed biotite grains are phlogopitic and contain 3 to 4 wt. percent F. Though it was not observed in rocks with advanced argillic alteration, fluorite (~49 wt% F) is also a common gangue mineral in the Resolution deposit (Schwarz, 2007). 25 Montmorillonite: The PIMA™ spectrometer identified the presence of montmorillonite as frequently as the other clay minerals and sericite, including in feldspar sites that were soft but not necessarily pyrite-bearing. Abundances of montmorillonite were not compiled in this study given the lack of evidence for association with advanced argillic or sericitic alteration. Although it is possible that this PIMA™ determination is spurious, an alternative is that montmorillonite was formed as a late, weak hydrolytic overprint at low temperatures, postdating deposition of all or most sulfides. Pyrite: Abundances of sulfide minerals were estimated visually during logging of core and are summarized in terms of relative abundances (Fig. 13). Large volumes of rock contain high pyrite abundances, which are observed within rocks with advanced argillic alteration and, to a lesser degree, with sericitically altered rocks (Figs. 3 and 13). Where pyrite occurs with other sulfides, petrographic observations of sulfide textures suggest that pyrite was deposited earliest relative to other sulfides (Fig. 16A-C). Pyrite is commonly observed as angular, brecciated grains and as rounded grains cemented in bornite-chalcocite-digenite or chalcopyrite-bornite. Bornite and chalcocite: Supergene chalcocite occurs only locally within the leached cap (Manske and Paul, 2002). Hypogene examples of both chalcocite and digenite are well documented at Resolution (e.g., Manske and Paul, 2002, p. 212). Chalcocite and digenite commonly are intergrown with one another and with bornite (Fig. 16B,C); in addition, there are uncommon occurrences of hypogene covellite (Harrison, 2007), which was not logged in this 26 study. Indeed, electron microprobe analyses by Harrison (2007) reveal the presence of a variety of Cu-S phases of various stoichiometries, as well as various compositions of bornite. As noted by earlier workers (Troutman, 2001; Manske and Paul, 2002; Harrison, 2007), petrographic evidence suggests that bornite commonly was precipitated around earlier grains of pyrite and fills cracks in and locally partially replaces pyrite (Fig. 16A-C). Chalcocite appears to be stable with pyrite, though not necessarily coprecipitated with it (Fig. 16B,C). In this study, the term chalcocite may be used for any of the Cu-S phases. Given the common intimate intergrowth of bornite and chalcocite, visual estimates of abundance were recorded for the sum of bornite and chalcocite (Fig. 14). The highest bornite + chalcocite values are observed within advanced argillic altered regions and to a lesser extent in rocks assigned to the sericitic and potassic/sericitic zones in the RCC alteration block model. Resolution geologists have observed that the highest concentrations of bornite occur at a fairly distinct level at an elevation of ~ -500m, which is near the base of sericitic alteration with 7-14 vol% pyrite but outside the highest zone of pyrite. In contrast, the most abundant chalcocite tends to occur at or slightly above the region of most abundant bornite in the most pyritic rocks. Chalcopyrite: As shown in Figure 15, high values of chalcopyrite are observed in advanced argillic, sericitic, and potassic/sericitic altered zones of the RCC alteration block model. The highest chalcopyrite values are observed consistently in Proterozoic diabase and Mescal Limestone and more locally in Cretaceous volcaniclastic rocks and porphyry, generally where altered to potassic assemblages and skarn, which were not the focus of this study. Most of the 27 other occurrences of chalcopyrite are associated with pyritic veins with sericitic alteration envelopes. No occurrences of chalcopyrite were observed that are unambiguously associated with advanced argillic assemblages. Veins and crosscutting relationships Mineralogy of veins: Many types of hypogene veins were observed and logged, and photographs of key examples are illustrated in Figure 17. In some cases, the vein filling and the alteration envelope are distinct; in other cases, they are not, especially where the outer edges of the envelopes cannot be determined because of a high density of superimposed veins. Similar veins were grouped into a smaller number of types (Table 3). Crosscutting relationships: Crosscutting relationships between veins were recorded and documented during logging, with attempts to avoid potentially deceiving exposures. Figure 18 shows photographs of representative observed crosscutting relationships between veins, and the petrographic relationships also provide paragenetic constraints (Fig. 16). Table 3 provides a matrix tabulation of all observations from this study, which focused on a part of the deposit with abundant sericitic and advanced argillic alteration and thus lacks exposures of many higher temperature or earlier veins. The table shows that the general paragenesis of veins in the deposit from older to younger is quartz - molybdenite veins (which may lack alteration envelopes but generally occur in areas of potassic alteration), through veins related to sericitic alteration, transitional sericitic-advanced argillic assemblages (e.g., Fig. 16H), and uncommon pyrophylliteand alunite-bearing assemblages, and finally kaolinitic clay-bearing advanced argillic 28 assemblages (Fig. 16D). The data are consistent with the observations of Marsh (2002) that topaz-bearing veins probably formed broadly contemporaneous with pyrophyllite and kaolinite. There are a few observations to the lower left of the diagonal of the matrix in Table 3, which is the field of possible reversals in the vein paragenesis. (See Seedorff and Einaudi, 2004a, for explanation of terminology regarding reversals and normal and anomalous types of crosscutting relationships.) Because the detailed paragenesis of veins is only partially constrained by the relatively limited number of observations in Table 3, the apparent reversals that involve different veins within the same silicate alteration type (e.g., those advanced argillic veins in the lower right part of the matrix) probably are not significant. The other apparent reversals are isolated observations that could represent spurious observations because of cryptic, unrecognized deceiving exposures or may represent local reversals. In any case, no definitive evidence has been provided to date for multiple mineralizing events or major reversals in the sequence of crosscutting veins (Troutman, 2001; Harrison, 2007; A. Schwarz, oral comm., 2007). Orientations of veins: A variety of data exist regarding vein orientations at Resolution. The staff geologists collect extensive data on vein orientation based on measurements on oriented core their correlation with down hole data. One such tool, Wellcad, is a core orientation program that collects both bore hole televiewer and acoustic resonance imaging data. The program collects copious amounts of information regarding vein fracture and fault orientations. An RCC internal technical report (Trout, 2009) that summarizes the vein database reports an overall vein trend of N30E; the earliest, quartz – molybdenum veins and veins within the 29 porphyry are reported to have a northwesterly strike; chalcopyrite veins strike west; and chalcocite- and bornite-bearing veins follow a N30E trend. In an earlier study, Troutman (2001) notes a dominant strike of N55E with southeasterly dips in the northern part of the deposit and northwesterly dips in the southern part of the deposit for veins associated with sericitic and advanced argillic alteration. In this study, vein orientations to core axis were measured (Table 4). Because the portions of the holes logged in this study are nearly vertical, 90 minus the angle to core axis is the approximate dip of the vein. These data show that the dips on sericitic veins vary widely, whereas for veins associated with advanced argillic alteration the orientations are more systematic, with the angle to core axis generally varying from 0 to 30 degrees, i.e., the present dip of the veins (i.e., after tilting associated with normal faulting) is steep at ~60-90°. The data of Troutman (2001) and the Wellcad database suggest that these veins have northeasterly strikes. Interpretations Characterization and classification of assemblages into silicate alteration types As in many deposits where advanced argillic alteration is developed, the assignment of alteration products at Resolution to hydrothermal mineral assemblages is complicated by the widespread evidence for superposition of events, the difficulty in some cases of relating alteration features to particular veins or fluid channels, and the fine-grained nature of many of the products of hydrolytic alteration. In spite of hundreds of PIMA and UV light determinations, 30 supplemented by petrography and electron microprobe analyses, the ability to identify hydrothermal mineral assemblages with certainty continuously down a given drill hole remains impossible’ nonetheless, it is possible to do this discontinuously in many drill holes. The information gathered from such intervals, as illustrated in Figure 19, can be synthesized to construct a list of the various mineral assemblages produced by acidic fluids at Resolution (Table 5), even if the assignments are less certain than they might be in other deposits or other parts of the Resolution deposit, and to assign them to silicate alteration types using the criteria of Seedorff et al. (2005a). Interpretations rely heavily on the most definitive cases, such as compelling cases for coprecipitated minerals filling veins (Fig. 17) and petrographic textural evidence observed here (Fig. 16) and documented in other works (e.g., Troutman, 2001; Harrison, 2007). Given the difficulty of this effort, Table 5 also offers guidance as to the confidence in each proposed assemblage and an interpretation of the relative abundance of each assemblage. Most of the assemblages observed (Table 5) either (1) are of the sericitic type (Figs. 16E, 19D,G), (2) are transitional from sericitic to advanced argillic types (Figs. 16H,19E), or (3) are of the advanced argillic type (Figs. 16F,G, 19A-C,H). The intensity of advanced argillic alteration at Resolution is largely governed by the abundance of kaolinite and dickite (Fig. 9), given the relative lack of pyrophyllite (Fig. 12) and rarity of andalusite. Among other aluminous minerals, such as topaz (Fig. 11), alunite (Fig. 12), and zunyite (Fig. 12), that can be present in advanced argillic alteration, only topaz is fairly abundant at Resolution. The geochemical stabilities of these minerals do not require that the 31 former three minerals form only under advanced argillic conditions (e.g., Barton, 1982; Seedorff, 1986; Seedorff et al., 2005a), and some of these minerals clearly have formed in other deposits in the sericitic or potassic environments (e.g., Seedorff and Einaudi, 2004a, b). At Resolution, hand specimen and petrographic observations indicate that topaz in many cases clearly formed in equilibrium with kaolinite but not with K-feldspar or sericite, although certain quartz-topaz occurrences are not diagnostic. Nonetheless, the fluorine-poor nature of all topaz grains analyzed (XF-Topaz of ~0.6) coupled with phase relations (e.g., Barton, 1982; Seedorff and Einaudi 2004b) indicate that topaz at Resolution probably formed mostly in the advanced argillic environment. The close association of alunite with pyrophyllite at Resolution also indicates that the few alunite occurrences known from Resolution also formed in the advanced argillic environment. Relationship between silicate alteration assemblages and opaque assemblages At Resolution, the deeper part of the ore body characterized by potassic alteration (e.g., Manske and Paul, 2002) contains opaque assemblages of chalcopyrite, chalcopyrite + magnetite, and chalcopyrite + pyrite, i.e. intermediate sulfidation state assemblages (Einaudi et al., 2003). As shown in Table 5, a sericitic assemblage with relatively low abundance is characterized by the intermediate sulfidation state opaque assemblage of chalcopyrite + pyrite. The most abundant sericitic assemblages, however, are high-sulfidation state assemblages with pyrite, bornite, and chalcocite (Fig. 19D,G), confirming earlier conclusions of Troutman (2001) and Manske and Paul (2002) regarding Resolution but differing from observations in many other porphyry systems (e.g., Einaudi, 1982). High-sulfidation state assemblages persist through transitional 32 advanced argillic-sericitic and advanced argillic assemblages (Table 5). Indeed, three polished thin sections in this study (e.g., Fig. 19D) contain bornite–chalcocite with sericite but lack kaolinite, topaz, and/or alunite; nonetheless, the majority of polished thin sections contain bornite-chalcocite stable with clay and/or topaz. The highest copper grades (>3%) coincide generally with high abundances of bornite-chalcocite-associated exclusively with zones of intense advanced argillic alteration and high pyrite content (Figs. 4, 7, 9, 11, 13, 14). As noted above, enargite is uncommon in high-sulfidation mineral assemblages, as local spikes in arsenic content correspond primarily with occurrences of arsenic-rich pyrite (Fig. 6). The very-high sulfidation state assemblage covellite + pyrite, also associated with advanced argillic alteration, has been reported locally at Resolution (e.g., Harrison, 2007) but was not observed in this study. Evolutionary paths of fluids The succession of mineral assemblages (Table 5) with time as documented by crosscutting relationships (Fig. 18, Table 3), supplemented by petrographic observations (Fig. 16), can be used to deduce evolutionary paths of hydrothermal fluids that can be displayed as paths across phase diagrams. Although some portions of a path may represent progressive evolution of a single batch of hydrothermal fluid as it reacts with wall rock, new inputs of fluid, perhaps varying compositions, may also occur with time. The sequence of opaque assemblages (Table 5) documented above constitutes an evolutionary path of increasing sulfidation state with time in the region of the Resolution deposit examined in this study. This path probably corresponds to a segment of the gently 33 upward-inclined arc of the looping path on a T-fS2 diagram commonly observed in porphyry-related systems that attain high and very high sulfidation states (e.g., Einaudi et al., 2003). Changes in the acidity of the fluid with decreasing temperature are illustrated schematically in Figure 20. Without better constraints on temperature (e.g., from fluid inclusion data), a precise path cannot be shown, but the sequence of silicate phases is nonetheless indicative of a general trajectory based on relative ages of assemblages. The sequence of veins and mineral assemblages (Tables 3 and 5) indicate that earlier, presumably hotter, fluids were stable with K-feldspar and/or biotite, producing potassic alteration. Through time, probably associated with a decline in temperature, the fluid became stable with sericite, although the potassium-poor compositions of sericite at Resolution (Table 1) suggest that much of this sericite was produced at fairly low temperatures, approaching or within the nominal stability of illite (Fig. 20). The abundance of kaolinite-dickite and the rarity of pyrophyllite suggest that the path may have left the sericite field and entered the field of aluminosilicate minerals at fairly low temperatures (~300ºC) in the vicinity of the pyrophyllite-kaolinite boundary (Fig. 20), coinciding with the transition from sericitic to advanced argillic alteration. The absence of observations of diaspore and corundum and the silicification commonly associated with kaolinite (e.g., Troutman, 2001) indicate that the fluid probably was quartz-saturated during advanced argillic alteration at Resolution (see also Hemley et al., 1980). If the PIMA™ identifications of montmorillonite and the suggestion that they might represent a late sulfide-absent alteration product are valid (see 34 above), then the fluid, after producing abundant kaolinite, may finally have become less acid at very late stages (Fig. 20). The path can be compared with other possible paths that might produce advanced argillic alteration somewhere during their evolution (e.g., Fig. 12 of Seedorff et al, 2005a). The presence of topaz and its composition offers further insight into the geochemical evolution of the fluid, as displayed on diagrams involving temperature and activities of K+ and F(Figs. 21, 22). Topaz is commonly observed in association with kaolinite/dickite (Table 5); hence, the general path of the fluid on these diagrams interpreted to have traversed from the sericite or muscovite field (without topaz) to a position straddling the topaz-kaolinite boundary while topaz was deposited. The fluorine-poor compositions of topaz solid solution (Table 2) are consistent with formation of topaz during advanced argillic alteration at relatively low temperatures of ~300ºC (Figs. 21, 22). The suggestion that at least some of the kaolinite and dickite postdate topaz (with kaolinite) indicates that the path then headed into the kaolinite-only field (Fig. 22), perhaps then veering toward higher values of K+/H+ at late stages (if montmorillonite formed late, see above; Fig. 22). The paths of Figures 21 and 22 are markedly different than those that produced topaz during potassic and sericitic alteration at Henderson (e.g., Figs. 9, 10, 12 of Seedorff and Einaudi, 2004b). 35 Discussion Geometry and characterization of sericitic and advanced alteration The distribution of sericitic and advanced argillic alteration in porphyry deposits is variable (e.g., Fig. 10 of Seedorff et al., 2005). In some deposits, advanced argillic alteration largely is barren (e.g., the lithocap of Sillitoe, 2010), whereas in many systems it can be well mineralized (e.g., Einaudi et al., 2003; Seedorff et al., 2005). Likewise, there also can be a general vertical progression from high to low temperature advanced argillic alteration from deeper to shallow levels, i.e., andalusite to pyrophyllite to kaolinite (e.g., Gustafson and Hunt, 1975; Watanabe and Hedenquist, 2001). Rocks exhibiting sericitic alteration and advanced argillic alteration at Resolution generally are mineralized and commonly exhibit high grades and thus do not constitute a barren lithocap at the preserved levels. Although some uncertainty remains because the top of the Resolution system has been eroded, the preserved and drilled portion of the pattern suggests that advanced argillic alteration is commonly enveloped in three dimensions by a thick rind of sericitic alteration, although in places advanced argillic alteration extended downward into potassic alteration and may have extended as a pipe or funnel locally upward through the sericitic rind at levels above the Tertiary erosion surface (Figs. 3, 9, 10). One could regard the preserved part of Resolution to be the “root” of a zone of advanced argillic alteration, to the extent that some rocks affected by advanced argillic alteration have been eroded, yet it is notable that the preserved “root” is overwhelming dominated by kaolinite and dickite, rather than either 36 pyrophyllite or andalusite. Moreover, the relatively potassium-poor compositions of sericite at Resolution (Table 1) indicates that the uneroded part of the Resolution system that was the subject of this study also is not the root of sericitic alteration, but rather probably its uppermost branches. Hence, it seems unlikely that much of the top of the system has been eroded. Resolution represents an interesting variant on many possible geometries of hydrolytic alteration in porphyry systems. The sericitic to advanced argillic transition Although the identities, distributions, and relative ages of mineral assemblages is crucial to understanding the geochemical environment of alteration-mineralization and the dynamics of hydrothermal systems (e.g., Seedorff et al., 2005a), rocks exhibiting hydrolytic alteration commonly represent a special challenge in identifying mineral assemblages and in many cases is been regarded as virtually impossible (e.g., Khashgerel et al., 2006). The difficulty is that the silicate minerals commonly are fine-grained and light colored and difficult to identify with the naked eye, hand lens, or in some cases even petrographically, such as distinguishing between sericite and pyrophyllite. Other techniques, such as infrared spectrometers and X-ray diffraction, aid in mineral identification, but the scale resolution of such determinations commonly results in the loss of the textural relationships necessary to establish that the minerals formed contemporaneously in apparent equilibrium. This study represents one of the few attempts to determine mineral assemblages in areas of intense hydrolytic alteration and their relative ages (cf., Lipske and Dilles, 2000; Khashgerel 37 et al., 2009). At Resolution, numerous advanced argillic assemblages are present (Table 5), though most contain kaolinite or dickite, and topaz is abundant. The predominance of kaolinite contrasts with deposits such as Butte and El Salvador, where andalusite and pyrophyllite are much more common (e.g., Meyer et al., 1968; Howard, 1972; Brimhall, 1977; Gustafson and Hunt, 1975; Watanabe and Hedenquist, 2001; Field et al, 2005; Rusk et al., 2008). Presence of topaz and other fluorine-bearing minerals Topaz forms in a wide range of geochemical environments (e.g., Barton, 1982). In certain porphyry molybdenum and tungsten systems, it forms during potassic and sericitic alteration, but in porphyry copper deposits it forms almost exclusively during advanced argillic alteration (Seedorff, 1986; Seedorff and Einaudi, 2004b). Metallurgical tests indicate that Resolution is a relatively fluorine-rich deposit (Schwarz, 2007), although the spatial distribution of fluorine at Resolution is not well known because fluorine is not routinely assayed. Microprobe analyses conducted in this study (Table 2) indicate that the most important fluorine-bearing minerals throughout the deposit likely are biotite (~3-4 wt% F), topaz (~11-12 wt% F), fluorite (~49 wt% F), and sericite (~1 wt% F), and other fluorine-bearing phases also are locally present (e.g., zunyite, 6-7 wt% F). As in other porphyry copper systems, topaz at Resolution formed during advanced argillic alteration but at relatively low temperatures with kaolinite, consistent with its relatively fluorine-poor composition (XF-Topaz = 0.58 – 0.64). The location and mineralogic host of fluorine in porphyry systems has potential practical 38 implications in processing and environmental storage (e.g., Pangum et al., 1997; Sutter, 2002). Arsenic abundance and mineralogy in porphyry-related systems For those porphyry systems that contain considerable arsenic, arsenic is generally present in intermediate sulfidation state assemblages as tennantite and in high-sulfidation state assemblages as enargite and luzonite, which tend to be associated with advanced argillic alteration (e.g., Meyer et al., 1968; Einaudi, 1982; Einaudi et al., 2003). Even though the nearby Magma vein, containing both tennantite and enargite (Gustafson, 1961; Hammer and Peterson, 1968), is notably rich in arsenic, Resolution is relatively arsenic-poor in spite of widespread, intense advanced argillic alteration (e.g., Manske and Paul, 2002), a distinction shared with Oyu Tolgoi (Khashgerel et al., 2008, 2009). The upper part of the Resolution ore body is arsenic-bearing, but arsenic occurs most in solid solution in other sulfides (e.g., arsenic-bearing pyrite) rather than as enargite. The low arsenic contents of ores have metallurgical and environmental benefits to the project, but geochemical controls on arsenic content and mineralogy are not well understood. An elevated oxidation state of the fluid has been suggested as one possible cause (R. Beane, quoted in Manske and Paul, 2002). Relationship between silicate alteration types and sulfidation state of sulfides Coexisting minerals, mainly the opaque sulfide and oxide minerals, can be used to constrain the sulfidation state in which they formed (e.g., Barton, 1970; Barton and Skinner, 1967, 1979; Einaudi et al., 2003), and there is a long-recognized tendency for mineral 39 assemblages containing silicate minerals characteristic of advanced argillic alteration to contain sulfide minerals characteristic of high and very high sulfidation states, because reactions involving sulfur species that lead to higher sulfidation states also generate acid (e.g., Meyer and Hemley, 1967; Einaudi, 1982). At the Resolution deposit, most of the sericitic assemblages and all of the advanced argillic assemblages contain high sulfidation state opaque assemblage (Table 5). There is no a priori reason why coupled reactions involving sulfur species should cause the transition from sericitic to advanced argillic to coincide precisely with the transition from intermediate to high sulfidation states. Resolution is thus an exception to the general “rule” that high sulfidation-state minerals are deposited only during advanced argillic alteration, for which Chuquicamata is another possible example. Advanced argillic alteration is not present (or at least not yet reported) at exposed and drilled levels of Chuquicamata, in spite of the fact that high-sulfidation state sulfide minerals, including enargite, are widespread and vertically extensive, seemingly cogenetic with sericitic alteration (Ossandón et al., 2001). Source of high copper grades The search for genetic understanding of controls on metal grades are an enduring theme of economic geology, and Resolution is distinctive for having high hypogene copper grades in a variety of alteration types (Manske and Paul, 2002; Schwarz, 2007). Telescoping of alteration-mineralization events is also offered as one possible explanation for porphyry deposits 40 with higher hypogene grades (e.g., Sillitoe, 2010). The compositions of certain host rocks, such as carbonate rocks and diabase, also may have locally enhanced metal deposition (Fig. 4), but many porphyry deposits in the region are hosted by identical units yet have half the grade of Resolution, as noted by Manske and Paul (2002). Although some of the highest grades in the Resolution deposit occur in areas of advanced argillic alteration with abundant digenite (Figs. 4, 14) and superposition of later high-sulfidation on earlier intermediate sulfidation assemblages locally increased copper grades and probably added to the size of the ore body, the fact remains that areas of the deposit containing only intermediate sulfidation assemblages also exhibit high hypogene grades (Figs. 4, 15). These observations suggest that the principal control on high hypogene grades at Resolution may be high fluxes of copper-bearing hydrothermal fluid, which would have been dictated by conditions in the underlying magma chamber, rather than by conditions at the site of metal deposition. Resolution offers considerable opportunity for future work to yield a better understanding of whether or how hydrolytic alteration redistributed or added copper to the ore body. Conclusions Resolution is a geologically and economically significant example of porphyry copper mineralization with extensive development of both advanced argillic alteration and high-sulfidation state opaque assemblages. This study represents one of the few attempts to determine mineral assemblages in areas of intense hydrolytic alteration and their relative ages. 41 The deposit is an exception to the general “rule” that high sulfidation-state minerals are deposited only during advanced argillic alteration, as sericitic assemblages contain high-sulfidation state opaque assemblages. Numerous advanced argillic assemblages are present; most contain kaolinite or dickite, in contrast to deposits where andalusite and pyrophyllite are much more common. Resolution is a relatively fluorine-rich deposit, and topaz, formed during advanced argillic alteration, is abundant and predictably exhibits fluorine-poor compositions. Acknowledgments Support for this project was generously provided by Resolution Copper Mining LLC and by Science Foundation Arizona through the UA Lowell Institute for Mineral Resources. We are grateful for the opportunity to build on the work of Resolution geologists and earlier workers. During the early stages of the project, Adam Schwarz provided invaluable assistance in geologic logging, and Bill Hart provided invaluable aid by making the geologic and geochemical database more useful to us. We thank Ken Domanik for technical assistance with electron microprobe analyses. We acknowledge also benefitting, directly and indirectly over many years, from the observations and insights on the geology of the Superior district from Geoff Ballantyne, Marco Einaudi, Don Hammer, Kurt Friehauf, David Maher, Scott Manske, Tim Marsh, Alex Paul, and Sandra Troutman. 42 References Anonymous, 2008, Resolution Copper Mining LLC reports an Inferred Resource of over 1 billion tonnes at its property in Arizona, USA: Rio Tinto Press Release, 29 May 2009, 6 p., www.riotinto.com/media/5157_7821.asp, accessed 3 August 2010. Anonymous, 2010, Resolution Copper Mining--Who we are: Project profile, www.resolutioncopper.com/res/whoweare/1.html, accessed 3 August 2010. Arribas R., A., Jr., 1995, Characteristics of high-sulfidation epithermal deposits, and their relation to magmatic fluid, in Thompson, J.F.H., ed., Magmas, fluids, and ore deposits: Mineralogical Association of Canada Short Course, v. 23, p. 419-454. Arribas, A., Jr., Hedenquist, J. W., Itaya, T., Okada, T., Concepción, R. A., and Garcia, J. S., Jr., 1995, Contemporaneous formation of adjacent porphyry and epithermal Cu-Au deposits over 300 ka in northern Luzon, Philippines: Geology, v. 23, p. 337-340. Bailey, S.W., 1984, Classification and structures of the micas, in Bailey, S.W., ed., Micas: Mineralogical Society of America Reviews in Mineralogy, v. 13, p. 1-12. Ballantyne, G. H., Marsh, T. M., Hehnke, C., Andrews, D. S., Eichenlaub, A. B., and Krahulec, K. A., 2003, The Resolution copper deposit, a deep, high-grade porphyry copper deposit in the Superior district: Arizona: Marcofest Conference, 3-5 April 2003, Golden, Colorado, 2003, 12 p. Barton, M. D., 1982, The thermodynamic properties of topaz solid solutions and some petrologic applications: American Mineralogist, v. 67, p. 956-974. 43 Barton, M.D., Ilchik, R.P., and Marikos, M.A., 1991, Metasomatism, in Kerrick, D.M., ed., Contact metamorphism: Mineralogical Society of America Reviews in Mineralogy, v. 26, p. 321-350. Barton, P.B., Jr., 1970, Sulfide petrology, in Morgan, B.A., ed., Fiftieth anniversary symposia: Mineralogy and petrology of the upper mantle; sulfides; mineralogy and geochemistry of non-marine evaporites: Mineralogical Society of America Special Paper 3, p. 187-198. Barton, P.B., Jr., and Skinner, B.J., 1967, Sulfide mineral stabilities, in Barnes, H.L., ed., Geochemistry of hydrothermal ore deposits: New York, Holt, Rinehart, and Winston, p. 236-333. Barton, P.B., Jr., and Skinner, B.J., 1979, Sulfide mineral stabilities, in Barnes, H.L., ed., Geochemistry of hydrothermal ore deposits, 2nd Edition: New York, John Wiley and Sons, p. 278-403. Bodnar, R.J., and Beane, R.E., 1980, Temporal and spatial variations in hydrothermal fluid characteristics during vein filling in preore cover overlying deeply buried porphyry copper-type mineralization at Red Mountain, Arizona: Economic Geology, v. 75, p. 876-893. Brigatti, M. F., and Guggenheim, S., 2002, Mica crystal chemistry and the influence of pressure, temperature, and solid solution on atomistic models, in Mottana, A., Sassi, F. P., Thompson, J. B., Jr., and Guggenheim, S., eds., Micas: Crystal chemistry and metamorphic petrology: Reviews in Mineralogy and Geochemistry, v. 46, p. 1-97. 44 Brimhall, G.H., Jr., and Ghiorso, M.S., 1983, Origin and ore-forming consequences of the advanced argillic alteration process in hypogene environments by magmatic gas contamination of meteoric fluids: Economic Geology, v. 78, p. 73-90. Bryant, D.G., 1968, Intrusive breccias associated with ore, Warren (Bisbee) mining district, Arizona: Economic Geology, v. 63, p. 1-12. Chivas, A.R., 1978, Porphyry copper mineralization at the Koloula igneous complex, Guadalcanal, Solomon Islands: Economic Geology, v. 73, p. 645-677. Corn, R.M., 1975, Alteration-mineralization zoning, Red Mountain, Arizona: Economic Geology, v. 70, p. 1437-1447. Einaudi, M.T., 1982, Description of skarns associated with porphyry copper plutons, southwestern North America, in Titley, S.R., ed., Advances in geology of the porphyry copper deposits, southwestern North America: Tucson, University of Arizona Press, p. 139-183. Einaudi, M. T., Hedenquist, J. W., and Inan, E. E., 2003, Sulfidation state of fluids in active and extinct hydrothermal systems: Transitions from porphyry to epithermal environments, in Simmons, S. F., and Graham, I. J., eds., Volcanic, geothermal, and ore-forming fluids: Rulers and witnesses of processes within the Earth: Society of Economic Geologists Special Publication 10 (Giggenbach Volume), p. 285-313. Ferguson, C. A., Skotnicki, S. J., and McIntosh, W. C., 1998, Rapid extensional tectonism and short-lived volcanism along the Transition Zone, Basin and Range boundary, central Arizona [abs.]: Geological Society of America Abstracts with Programs, v. 30, no. 6, p. 9. 45 Field, C. W., Zhang, L., Dilles, J. H., Rye, R. O., and Reed, M. H., 2005, Sulfur and oxygen isotope record in sulfate and sulfide minerals of early, deep, pre-Main Stage porphyry Cu-Mo and late Main Stage base-metal mineral deposits, Butte district, Montana: Chemical Geology, v. 215, p. 61-93. Friehauf, K. C., 1998, Geology and geochemistry of porphyry-related, carbonate-hosted massive replacement Cu-Au deposits--A case study of the Superior district, Arizona: Unpublished Ph. D. thesis, Stanford University, 221 p. Guilbert, J.M., and Schafer, R.W., 1979, Preliminary geochemical characterization of muscovites in porphyry base-metal alteration assemblages, in Ridge, J.D., ed., Papers on mineral deposits of western North America, The International Association on the Genesis of Ore Deposits, Fifth Quadrennial Symposium, Proceedings, v. 2: Nevada Bureau of Mines and Geology Report 33, p. 57-68. Gustafson, L. B., 1961, Paragenesis and hypogene zoning at the Magma mine, Superior, Arizona: Unpublished Ph. D. thesis, Harvard University, 93 p. Gustafson, L. B., and Hunt, J. P., 1975, The porphyry copper deposit at El Salvador, Chile: Economic Geology, v. 70, p. 857-912. Hammer, D. F., and Peterson, D. W., 1968, Geology of the Magma mine area, Arizona, in Ridge, J. D., ed., Ore deposits of the United States, 1933-1967 (Graton-Sales Volume): New York, American Institute of Mining, Metallurgical, and Petroleum Engineers, v. 2, p. 1282-1310. 46 Harrison, R., 2007, The evolution of hydrothermal alteration-mineralisation events in the Resolution porphyry Cu-Mo deposit, Superior, Arizona: Unpublished M. S. thesis, University of Bristol, England, 43 p. Harvey, B.A., Myers, S.A., and Klein, T.G., 1999, Yanacocha gold district, northern Peru, in Weber, G., ed., PACRIM '99, Proceedings: Australasian Institute of Mining and Metallurgy Congress, Bali, Indonesia, 10-13 October 1999, p. 445-459. Hedenquist, J.W., and Lowenstern, J.B., 1994, The role of magmas in the formation of hydrothermal ore deposits: Nature, v. 370, p. 519-527. Hemley, J. J., and Jones, W. R., 1964, Chemical aspects of hydrothermal alteration with emphasis on hydrogen metasomatism: Economic Geology, v. 59, p. 538-569. Hemley, J. J., Montoya, J. W., Marinenko, J. W., and Luce, R. W., 1980, Equilibria in the system Al2O3-SiO2-H2O and some general implications for alteration/mineralization processes: Economic Geology, v. 75, p. 210-228. Howard, K. L., Jr., 1972, Pyrophyllite-topaz alteration in the ore deposit at Butte, Montana: Unpublished Ph. D. Dissertation, University of California, Berkeley, 162 p. Huntington, J., and Yang, K., 2009, Interpretation of HyLogging data of coarse reject drill samples from Resolution Copper Project, Arizona: Unpublished pilot study report, 40 p. Khashgerel, B.-E., Rye, R. O., Hedenquist, J. W., and Kavalieris, I., 2006, Geology and reconnaissance stable isotope study of the Oyu Tolgoi porphyry Cu-Au system, South Gobi, Mongolia: Economic Geology, v. 101, p. 503-522. 47 Khashgerel, B.-E., Kavalieris, I., and Hayashi, K.-i., 2008, Mineralogy, textures, and whole-rock geochemistry of advanced argillic alteration: Hugo Dummett porphyry Cu-Au deposit, Oyu Tolgoi mineral district, Mongolia: Mineralium Deposita, v. 43, p. 913-932. Khashgerel, B.-E., Rye, R. O., Kavalieris, I., and Hayashi, K.-i., 2009, The sericitic and advanced argillic transition: Stable isotope and mineralogical characteristics from the Hugo Dummett porphyry Cu-Au deposit, Oyu Tolgoi district, Mongolia: Economic Geology, v. 104, p. 1087-1110. Lang, J.R., and Titley, S.R., 1998, Isotopic and geochemical characteristics of Laramide magmatic systems in Arizona and implications for the genesis of porphyry copper deposits: Economic Geology, v. 93, p. 138-170. Lipske, J.L., and Dilles, J.H., 2000, Advanced argillic and sericitic alteration in the subvolcanic environment of the Yerington porphyry copper system, Buckskin Range, Nevada, in Dilles, J.H., Barton, M.D., Johnson, D.A., Proffett, J.M., Jr., and Einaudi, M.T., eds., Part I. Contrasting styles of intrusion-associated hydrothermal systems: Society of Economic Geologists Guidebook Series, v. 32, p. 91-99. Maher, D. J., 2008, Reconstruction of middle Tertiary extension and Laramide porphyry copper systems, east-central Arizona: Unpublished Ph. D. thesis, University of Arizona, 328 p. Manske, S. L., and Paul, A. H., 2002, Geology of a major new porphyry copper center in the Superior (Pioneer) district, Arizona: Economic Geology, v. 97, p. 197-220. 48 Marsh, T. M., 2002, Recognition, distribution, timing, and exploration significance of topaz alteration at the Resolution project, Arizona, Kennecott Exploration Memorandum to Dave Andrews and Geoff Ballantyne, 16 April 2002, 7 p. McIntosh, W. C., and Ferguson, C. A., 1998, Sanidine, single crystal, laser-fusion 40Ar/39Ar geochronology database for the Superstition volcanic field, central Arizona: Arizona Geological Survey Open-File Report 98-27, 74 p. Meyer, C., and Hemley, J.J., 1967, Wall rock alteration, in Barnes, H.L., ed., Geochemistry of hydrothermal ore deposits: New York, Holt, Rinehart, and Winston, p. 166-235. Meyer, C., Shea, E. P., Goddard, C. C., Jr., and staff, 1968, Ore deposits at Butte, Montana, in Ridge, J. D., ed., Ore deposits of the United States, 1933-1967 (Graton-Sales Volume): New York, American Institute of Mining, Metallurgical, and Petroleum Engineers, v. 2, p. 1373-1416. Montoya, J. W., and Hemley, J. J., 1975, Activity relations and stabilities in alkali feldspar and mica alteration reactions: Economic Geology, v. 70, p. 577-583. Ossandón C., G., Fréraut C., R., Gustafson, L. B., Lindsay, D. D., and Zentilli, M., 2001, Geology of the Chuquicamata mine: A progress report: Economic Geology, v. 96, p. 249-270. Pangum, L.S., Manlapig, E.V., Mackinnon, I.D.R., and Lauder, D.W., 1997, Flotation process response of Ok Tedi fluorine bearing minerals, in Hancock, G.E., ed., PNG Geology, Exploration and Mining Conference, Madang, Papua New Guinea, 1997, Proceedings: Melbourne, Australasian Institute of Mining and Metallurgy, p. 173-180. 49 Pareja, G. A., 1998, The gold-rich jasperoids of the Superior district, Arizona: Controls on their location, and their relationship to porphyry-related carbonate-hosted massive sulfide bodies: Unpublished Ph. D. dissertation, Stanford University, Stanford, California, 213 p. Parry, W.T., Ballantyne, J.M., and Jacobs, D.C., 1984, Geochemistry of hydrothermal sericite from Roosevelt Hot Springs and the Tintic and Santa Rita porphyry copper systems: Economic Geology, v. 79, p. 72-86. Paul, A. H., and Knight, M. J., 1995, Replacement ores in the Magma mine, Superior, Arizona, in Pierce, F. W., and Bolm, J. G., eds., Porphyry copper deposits of the American Cordillera: Arizona Geological Society Digest 20, p. 366-372. Paul, A. H., and Manske, S. L., 2005, History of exploration at the Magma mine, Superior, Arizona, in Rhoden, H. N., Steininger, R. C., and Vikre, P. G., eds., Geological Society of Nevada Symposium 2005, Window to the World, Reno, Nevada, May 2005, Proceedings, v. 1, p. 629-638. Peterson, D. W., 1969, Geologic map of the Superior quadrangle, Pinal County, Arizona: U. S. Geological Survey Geologic Quadrangle Map GQ-818, scale 1: 24,000. Peterson, D. W., 1979, Significance of the flattening of pumice fragments in ash-flow tuffs, in Chapin, C. E., and Elston, W. E., eds., Ash-flow tuffs: Geological Society of America Special Paper 180, p. 195-204. 50 Reed, M. H., and Meyer, C., 1999, The Butte, Montana Main Stage vein system: Structural style, alteration, and mineral zoning, and their relationship to pre-Main Stage sericitic alteration [abs.]: Geological Society of America Abstracts with Programs, v. 31, no. 7, p. A382. Rusk, B. G., Miller, B. J., and Reed, M. H., 2008, Fluid-inclusion evidence for the formation of Main Stage polymetallic base-metal veins, Butte, Montana, USA, in Spencer, J. E., and Titley, S. R., eds., Ores and orogenesis: Circum-Pacific tectonics, geologic evolution, and ore deposits: Arizona Geological Society Digest 22, p. 573-581. Schwarz, A., 2007, Geology of the Resolution porphyry Cu-Mo system [abs.]: Ores and orogenesis: A symposium honoring the career of William R. Dickinson: Arizona Geological Society, Tucson, September 2007, Program with Abstracts, p. 83-84. Seedorff, E., 1986, Topaz in hydrothermal ore deposits: Advanced argillic, sericitic, or potassic alteration? [abs.]: Geological Society of America Abstracts with Programs, v. 18, p. 744. Seedorff, E., and Einaudi, M.T., 2004a, Henderson porphyry molybdenum system, Colorado: I. Sequence and abundance of hydrothermal mineral assemblages, flow paths of evolving fluids, and evolutionary style: Economic Geology, v. 99, p. 3-37. Seedorff, E., and Einaudi, M.T., 2004b, Henderson porphyry molybdenum system, Colorado: II. Decoupling of introduction and deposition of metals during geochemical evolution of hydrothermal fluids: Economic Geology, v. 99, p. 39-72. Seedorff, E., Dilles, J.H., Proffett, J.M., Jr., Einaudi, M.T., Zurcher, L., Stavast, W.J.A., Johnson, D.A., and Barton, M.D., 2005a, Porphyry deposits: Characteristics and origin of hypogene 51 features, in Hedenquist, J.W., Thompson, J.F.H., Goldfarb, R.J., and Richards, J.P., eds., Economic Geology 100th Anniversary Volume, p. 251-298. Seedorff, E., Barton, M.D., Gehrels, G.E., Johnson, D.A., Maher, D.J., Stavast, W.J.A., and Flesch, E., 2005b, Implications of new U-Pb dates from porphyry copper-related plutons in the Superior-Globe-Ray-Christmas area, Arizona [abs.]: Geological Society of America Abstracts with Programs, v. 37, no. 7, p. 164. Seedorff, E., Barton, M.D., Stavast, W.J.A., and Maher, D.J., 2008, Root zones of porphyry systems: Extending the porphyry model to depth: Economic Geology, v. 103, p. 939-956. Short, M. N., Galbraith, F. W., Harshman, E. N., Kuhn, T. H., and Wilson, E. D., 1943, Geology and ore deposits of the Superior mining area, Arizona: Arizona Bureau of Mines and Geology Series no. 16, Bulletin 151, 159 p. Sillitoe, R.H., 2010, Porphyry copper systems: Economic Geology, v. 105, p. 3-41. Sillitoe, R.H., and Hedenquist, J.W., 2003, Linkages between volcanotectonic settings, ore-fluid compositions, and epithermal precious metal deposits, in Simmons, S.F., and Graham, I.J., eds., Volcanic, geothermal, and ore-forming fluids: Rulers and witnesses of processes within the Earth: Society of Economic Geologists Special Publication 10 (Giggenbach Volume), p. 315-343. Stavast, W.J.A., Butler, B.F., Seedorff, E., Barton, M.D., and Ferguson, C.A., 2008, Tertiary tilting and dismemberment of the Laramide arc and related hydrothermal systems, Sierrita Mountains, Arizona: Economic Geology, v. 103, p. 629-636. 52 Sutter, J.W., 2002, Hydrofluoric acid generation in pyritic mine dumps and tailings [M. S. thesis]: Tempe, Arizona State University, 78 p. Titley, S.R., 1982, Geologic setting of porphyry copper deposits, in Titley, S.R., ed., Advances in geology of the porphyry copper deposits, southwestern North America: Tucson, Arizona, University of Arizona Press, p. 37-58. Trout, C., 2009, Creation of a cemented joints database and analysis of the orientation and distribution of cemented joints within the Resolution porphyry copper deposit: Resolution Copper Mining Technical Report to Carl Hehnke, 14 August 2009, 22 p. Troutman, S. M., 2001, Advanced argillic alteration in the deeply buried Magma Porphyry Cu-Mo prospect, Superior, Arizona: Unpublished M. S. thesis, Stanford University, 115 p. Watanabe, Y., and Hedenquist, J. W., 2001, Mineralogic and stable isotopic zonation at the surface over the El Salvador porphyry copper deposit, Chile: Economic Geology, v. 96, p. 1775-1797. Wilkins, J., Jr., and Heidrick, T.L., 1995, Post-Laramide extension and rotation of porphyry copper deposits, southwestern United States, in Pierce, F.W., and Bolm, J.G., eds., Porphyry copper deposits of the American Cordillera: Arizona Geological Society Digest 20, p. 109-127. 53 Wrucke, C. T., 1989, The Middle Proterozoic Apache Group, Troy Quartzite, and associated diabase of Arizona, in Jenney, J. P., and Reynolds, S. J., eds., Geologic evolution of Arizona: Arizona Geological Society Digest 17, p. 239-258. 54 Tables Table 1. Representative Electron Microprobe Analyses of Sheet Silicate Minerals Table 2. Representative Electron Microprobe Analyses of Topaz and Zunyite Table 3. Mineralogy of Veins and Matrix of Crosscutting Relationships Table 4. Orientations of Common Vein Types Grouped by Assemblage Table 5. Mineral Assemblages Produced by Acidic Fluids at Resolution 55 Figure Captions Fig. 1. Location map. Map showing simplified surface geology of the Superior district, with location of lines of cross section in this study, modified from Manske and Paul (2002). Inset map shows location of Resolution deposit in state of Arizona. Fig. 2 (top). Cross sections showing distribution of rock types, based on Resolution block model of 2009. A. East-west cross section. B. North-south cross section. Fig. 3 (bottom). Cross sections showing distribution of generalized alteration, based on Resolution block model of 2009. A. East-west cross section. B. North-south cross section. Fig. 4 (top). Cross sections showing distribution of copper, based on contouring of copper assays from Resolution drill hole database. A. East-west cross section. B. North-south cross section. Fig. 5 (bottom). Cross sections showing distribution of arsenic, based on contouring of arsenic assays from Resolution drill hole database. A. East-west cross section. B. North-south cross section. Fig. 6. SEM images showing backscattered electron images of sulfide minerals (A, C, and E) and corresponding distribution of arsenic (B, D, and F) from samples collected from sulfide-rich 56 portions of assay intervals containing relatively high levels of arsenic. A, B: R1A-1314, chalcopyrite and chalcocite with pyrite. C, D: R3-1922, chalcopyrite and chalcocite with pyrite. E, F: R3-1604, chalcocite and pyrite. Note uniform, low-level distribution of arsenic, indicating presence of arsenic ion solid solution and lack of evidence for arsenic minerals such as tennantite or enargite. Fig. 7 (top). Cross sections showing distribution of iron, based on contouring of iron assays from Resolution drill hole database. A. East-west cross section. B. North-south cross section. Fig. 8 (bottom). Cross sections showing distribution of sulfur, based on contouring of sulfur assays from Resolution drill hole database. A. East-west cross section. B. North-south cross section. Fig. 9 (top). Cross sections showing distribution of clay, including kaolinite and dickite. A. East-west cross section. B. North-south cross section. Fig. 10 (bottom). Cross sections showing distribution of sericite. A. East-west cross section. B. North-south cross section. Fig. 11 (top). Cross sections showing distribution of topaz. A. East-west cross section. B. 57 North-south cross section. Fig. 12 (bottom). Cross sections showing distributions of alunite, pyrophyllite, and zunyite. A. East-west cross section. B. North-south cross section. Fig. 13. Cross sections showing distribution of pyrite. A. East-west cross section. B. North-south cross section. Fig. 14 (top). Cross sections showing distribution of bornite + chalcocite, where chalcocite also includes digenite and similar phases. A. East-west cross section. B. North-south cross section. Fig. 15 (bottom). Cross sections showing distribution of chalcopyrite. A. East-west cross section. B. North-south cross section. Fig. 16. Photomicrographs of selected textures of hydrothermal minerals. A. (Sample R1A-1261) Rounded pyrite grains in contact with chalcopyrite being replaced by bornite and possibly pyrite. B. (Sample R1A-1314) Chalcocite after bornite with pyrite grains. C. (Sample R5H-1670) Chalcocite after bornite with pyrite clasts. D. (Sample R2A-1172) Advanced argillic alteration front: clay on left, sericite on right. E. (Sample R2A-1207) Typical quartz + sericite + pyrite assemblage; variations in grain size of sericite may be controlled by mineralogy or grain size of 58 original igneous minerals. F. (Sample R2A-1223) Alunite - clay vein; mainly alunite crystals visible in photo. G. (Sample R13-1905) Fine-grained topaz with quartz surrounding bornite, with (earlier?) quartz - sericite - pyrite assemblage on right. H. (Sample R13-1960) Quartz - sericite clay - pyrite assemblage. Note that sericite has replaced feldspar phenocrysts with clay present mainly in the groundmass; this is a case where clay (probably kaolinite) and sericite appear to be stable together, thus probably constituting a transitional advanced argillic-sericitic type of assemblage. Fig. 17. Photographs of selected hydrothermal vein types. Circles with notations in black ink are locations of PIMA™ infrared spectrometer analyses. A. (Sample R2A-1223) Late stage dickite vein with large envelope. Bornite mineralization surrounding. Arrow points to late dickite vein. B. (Sample R3-1165) Dickite + quartz + topaz + pyrite > bornite vein with quartz clay pyrite bornite filling and topaz envelope passing through sericitic alteration. C. (Sample R3-1175) Thick quartz + kaolinite + pyrite + pyrophyllite + bornite cutting through quartz + sericite + pyrite vein and sericitic wall rock. D. (Sample R3-1395) Quartz + kaolinite + dickite + topaz + pyrite + bornite vein. Note orientation that is at low angle to core axis, as with most clay veins. Circles show where the vein crosscuts a quartz + sericite + pyrite vein. E. (Sample R4-1624) Extremely thick quartz + kaolinite + dickite + pyrite + bornite + chalcocite vein, and note low angle to core axis. This region is relatively high in arsenic. F. (Sample R13-1915) Alunite and bornite blebs with quartz + kaolinite + alunite + pyrite + bornite vein on right side of photo. G. 59 (Sample R13-1960) Kaolinite and alunite veins passing through sericitically altered rock. H. (Sample R13-2027) Quartz + sericite + pyrite vein running parallel to quartz + dickite + pyrite + bornite + chalcocite vein; in this case, relative ages are unclear. Fig. 18. Photographs of selected crosscutting vein relationships , with veins paralleled by double-pointed colored arrows for reference. Circles with notations in black ink are locations of PIMA™ infrared spectrometer analyses. A. (Sample R3-1264) Alunite - clay hairline veinlet (pink arrow) cutting quartz + sericite + pyrite vein (red), which is cut by thick quartz + kaolinite + dickite + pyrite vein (green). B. (Sample R3-1275) Alunite + kaolinite+ quartz + bornite hairline veinlet (pink) cutting and offsetting quartz + sericite + pyrite veins (red). C. (Sample R3-1313) Quartz + kaolinite + dickite + pyrite + bornite + chalcocite vein (green) cutting quartz + sericite + pyrite vein (blue). D. (Sample R3-1875) Quartz + sericite + pyrite vein (red) cut by quartz + kaolinite + topaz + pyrite (yellow) and quartz + kaolinite + pyrite (green) veins. E. (Sample R3-1917) Quartz + sericite + pyrite > chalcopyrite vein (blue) cutting quartz + sericite + pyrite (pink) and quartz veins. F. (Sample R4-1885) Quartz + pyrite + kaolinite + dickite + topaz vein (yellow) cutting quartz + sericite + pyrite (red) and other quartz veins. G. (Sample R6D-1734) Quartz + dickite + pyrite hairline veinlet (green) cutting and offsetting quartz sericite pyrite vein (red). H. (Sample R6D-1800) Quartz + kaolinite + dickite + pyrite + bornite vein (green) cutting quartz + kaolinite + pyrite vein (green) cutting and offsetting two quartz + sericite + pyrite veins (red), which cut and offset an earlier quartz + sericite + pyrite vein (red). 60 Fig. 19. Photographs of selected hydrothermal mineral assemblages and associations. Circles with notations in black ink are locations of PIMA™ infrared spectrometer analyses. A. (Sample R1A-1261) Typical quartz + topaz + dickite + massive pyrite with high sulfidation copper minerals. B. (Sample R2A-1283) Quartz + kaolinite + dickite + alunite + pyrite + bornite + chalcocite vein passing heavily advanced argillic altered rock; PIMA™ determinations in wall rock are 55% kaolinite + 45% alunite (center) and 48% dickite + 30% kaolinite + 23% alunite (off right edge). C. (Sample R4-1863) High grade bornite and chalcocite in advanced argillic altered breccia with PIMA™ determinations for dickite, kaolinite, and halloysite. D. (Sample R5H-1761) Typical sericitically altered rock with high bornite + chalcocite grade and PIMA™ determination for muscovite. E. (Sample R5H-1830) Sericitically altered with dickite blebs, with PIMA™ determination for dickite and muscovite. F. (Sample R13-1928) Planar topaz + zunyite + bornite + chalcocite + pyrite vein passing through (earlier?) sericitically altered rock. Circle on bottom right is a PIMA™ determination for 63% zunyite + 37% topaz, whereas circles on top right and left are 100% muscovite. G. (Sample R13-2060) Quartz + sericite + pyrite + bornite + chalcocite assemblage with a PIMA™ determination of 100% muscovite. H. (Sample R17C-1683) Late stage dickite vein with quartz + topaz + pyrite + chalcocite > bornite. Circle on right is a PIMA™ determination for 76% halloysite + 15% topaz + 9% dickite. Fig. 20. Possible evolutionary path of fluids at Resolution as a function of temperature and 61 acidity. Phase diagram based on Hemley and Jones (1964), Montoya and Hemley (1975), and Hemley et al, (1980). Fig. 21. Possible polythermal evolutionary path of fluids at Resolution on phase diagram showing relative stability of topaz as a function of temperature. Diagram shows fields of stability of andalusite (ANDAL), pyrophyllite (PYROPH), kaolinite (KAOL), sericite or muscovite (MUSC), K-feldspar (KSPAR), and topaz (TOPAZ), with topaz field contoured for mole fraction of fluor-topaz in topaz solid solution. Path follows labeled dots and passes into topaz field only at low temperatures (solid dot) at ~300ºC, forming relatively fluorine-poor topaz (XF-Topaz ~ 0.6). Based on Fig. 12 of Seedorff and Einaudi (2004b). Fig. 22. Possible quasi-isothermal evolutionary path of fluids at Resolution on an activity-activity diagram showing relative stability of topaz. See caption of Figure 21 for abbreviations. Analogous to Fig. 9, but based on part of Fig. 12 of Seedorff and Einaudi, 2004b. 62 Figures Winant et al. Figure 1 63 Winant et al. Figures 2 (top) and 3 (bottom) 64 Winant et al. Figures 4 (top) and 5 (bottom) 65 Winant et al. Figure 6 66 Winant et al. Figures 7 (top) and 8 (bottom) 67 Winant et al. Figures 9 (top) and 10 (bottom) 68 Winant et al. Figures 11 (top) and 12 (bottom) 69 Winant et al. Figure 13 70 Winant et al. Figures 14 (top) and 15 (bottom) 71 Winant et al. Figure 16 72 Winant et al. Figure 17 73 Winant et al. Figure 18 74 Winant et al. Figure 19 75 Winant et al. Figure 20 76 Winant et al. Figure 21 77 Winant et al. Figure 22 78 Appendix: Microprobe results. 79 Raw weight percent Sample number #1 #2 #3 #4 #5 #6 #7 #8 #9 #10 #11 #12 #13 #14 #15 #16 #17 #18 #19 #20 #21 #22 #23 #24 #25 #26 #27 #28 #30 #31 #32 #36 #37 #38 #40 #41 #42 #43 #44 #45 #46 #47 #29 4-1667 4-1667 4-1667 4-1667 4-1667 4-1667 4-1667 4-1667 4-1667 3-1917 3-1917 3-1917 3-1917 3-1917 3-1917 3-1917 1A-1347 1A-1347 1A-1347 1A-1347 1A-1347 6D-1862 6D-1862 6D-1862 6D-1862 6D-1862 6D-1862 6D-1862 3-1647 3-1647 3-1746B (1746.5) 3-1746B (1746.5) 3-1746B (1746.5) 3-1746B (1746.5) 3-1746B (1746.5) 3-1746B (1746.5) 3-1746B (1746.5) 3-1746B (1746.5) 13-1928 13-1928 13-1928 17C-1683 3-1647 Comments F ser ser clay quartz stilb fam?: high ser ser ser ser biotite biotite biotite sericite sericite apatite biotite clay clay clay quartz clay ser ser ser large grained m clay on side of S well formed mu ser near edge clay clay clay topaz topaz mica topaz clay topaz topaz topaz zun zun clay ser Na 1.191 1.539 0.215 0.126 0.083 1.19 1.451 1.044 1.035 4.171 3.366 3.165 0.977 1.144 5.382 3.471 0.959 0.955 0.45 0 0.558 0.543 1.089 0.876 0.263 0.171 0.979 0.658 0.634 0.739 0.076 12.098 10.932 1.697 11.835 0.182 11.04 11.269 11.52 6.83 6.054 1.095 0.893 Mg 0.07 0.036 0.019 0.015 0.017 0.071 0.056 0.104 0.063 0.174 0.07 0.084 0.196 0.018 0.14 0.041 0 0 0.001 0 0.008 0.126 0.039 0.056 0.074 0.017 0.059 0.108 0.022 0.001 0.024 0.02 0 0.071 0.016 0.007 0.015 0.006 0 0.14 0.18 0.003 0.11 Al 0.864 0.761 0.095 0.005 0.172 0.802 1.374 0.967 0.646 11.379 10.546 9.92 1.39 1.498 0 11.203 0.012 0.003 0.006 0 0.013 0.787 1.675 1.115 0.301 0.015 0.918 0.742 0.133 0.033 0 0.014 0.081 1.3 0 0.007 0 0 0.01 0.01 0.005 0 0.587 18.13 20.002 19.785 0.032 3.501 19.262 18.181 18.161 19.662 7.746 9.194 9.224 16.276 17.03 0.019 8.8 20.898 20.219 21.525 0.057 20.871 18.273 16.775 17.33 19.862 21.038 18.123 19.057 19.711 20.601 20.925 28.728 29.182 17.568 29.365 20.892 29.473 28.342 30.53 29.74 30.472 20.615 19.245 Si Cl 23.6 24.589 22.021 44.211 42.335 23.907 24.136 23.035 23.279 19.305 18.314 17.948 21.517 25.254 0.034 19.068 23.062 22.749 22.918 43.175 22.623 22.816 23.343 22.729 22.27 22.333 23.752 23.382 22.449 22.805 23.423 14.966 14.644 24.025 14.721 22.733 14.562 15.646 14.94 10.62 10.62 22.306 22.83 K 0.015 0.006 0.069 0.004 0.006 0.02 0 0.012 0.002 0.071 0.051 0.042 0.003 0.002 0.009 0.035 0.01 0.002 0.034 0 0 0.01 0.01 0.032 0.001 0.023 0.014 0.003 0.027 0 0 0.015 0.012 0 0.011 0.02 0 0.004 0 2.27 2.501 0.017 0.002 Ca 4.854 2.446 0.89 0.028 1.783 4.151 4.505 5.448 5.024 7.579 6.716 7.134 6.862 3.458 0.012 6.587 0.014 0.024 0.006 0 0 7.773 4.465 4.961 6.072 0.035 5.4 5.755 1.207 0.295 0.029 0.01 0.003 4.366 0.023 0.096 0.008 0.004 0.01 0 0 0.012 6.184 Sc 0 0.025 0.035 0.003 0 0 0.004 0.007 0 0.012 0.004 0.004 0.041 0.043 39.669 0 0.018 0.043 0.015 0.018 0.106 0.019 0 0 0.001 0.015 0 0.009 0.009 0.01 0.001 0.009 0.016 0 0 0.005 0.004 0 0 0 0 0.018 0.008 Ti V 0.238 0.353 0.04 0.029 0.022 0.732 0.252 0.358 0.303 1.137 1.14 1.18 0.275 0.071 0 1.224 0.011 0.018 0.024 0.029 0.013 0.34 0.352 0.476 0.033 0 0.044 0 0.018 0.022 0.002 0 0.023 0.107 0 0 0.013 0 0 0.02 0 0 0.223 Cr Mn 0.048 0 0.001 0 0 0 0.022 0 0 0.117 0.164 0.198 0.022 0.022 0.263 0.239 0 0 0.021 0.004 0.008 0 0 0.033 0 0 0 0.004 0 0 0 0.004 0.017 0.016 0.012 0 0.016 0.016 0 0 0.002 0.013 0.003 Fe Cu 1.21 1.136 0.183 0 0.15 1.257 0.829 0.927 0.518 6.515 7.072 8.198 1.808 0.443 0.024 6.044 0.078 0.083 0.057 0.021 0.12 1.272 2.871 2.545 0.942 0.021 2.053 1.049 0.03 0.026 0.03 0.716 0.061 0.961 0.129 0 0.034 0.055 0.76 0 0.042 0.093 0.239 Zn Moles 18.998 22.99 24.312 26.982 28.086 F 0.062691 0.081009 0.011317 0.006632 0.004369 0.062638 0.076376 0.054953 0.054479 0.219549 0.177177 0.166596 0.051426 0.060217 0.283293 0.182703 0.050479 0.050268 0.023687 0 0.029372 0.028582 0.057322 0.04611 0.013844 0.009001 0.051532 0.034635 0.033372 0.038899 0.004 0.636804 0.575429 0.089325 0.62296 0.00958 0.581114 0.593168 0.60638 0.359512 0.318665 0.057638 0.047005 Na 0.003045 0.001566 0.000826 0.000652 0.000739 0.003088 0.002436 0.004524 0.00274 0.007569 0.003045 0.003654 0.008525 0.000783 0.00609 0.001783 0 0 4.35E-05 0 0.000348 0.005481 0.001696 0.002436 0.003219 0.000739 0.002566 0.004698 0.000957 4.35E-05 0.001044 0.00087 0 0.003088 0.000696 0.000304 0.000652 0.000261 0 0.00609 0.007829 0.00013 0.004785 Mg 0.035538 0.031301 0.003908 0.000206 0.007075 0.032988 0.056515 0.039775 0.026571 0.46804 0.433778 0.408029 0.057173 0.061616 0 0.460801 0.000494 0.000123 0.000247 0 0.000535 0.032371 0.068896 0.045862 0.012381 0.000617 0.037759 0.03052 0.005471 0.001357 0 0.000576 0.003332 0.053472 0 0.000288 0 0 0.000411 0.000411 0.000206 0 0.024144 Al 0.671929 0.741309 0.733267 0.001186 0.129753 0.713883 0.67382 0.673078 0.728708 0.28708 0.340746 0.341858 0.603217 0.631162 0.000704 0.326143 0.774516 0.749351 0.797754 0.002113 0.773516 0.677229 0.621711 0.64228 0.73612 0.779705 0.67167 0.706286 0.730524 0.763509 0.775517 1.06471 1.081536 0.651101 1.088318 0.774294 1.092321 1.050404 1.131495 1.102216 1.129345 0.764028 0.713253 Si 0.840276 0.87549 0.784056 1.574129 1.507335 0.851207 0.859361 0.82016 0.828847 0.687353 0.652069 0.639037 0.766111 0.899167 0.001211 0.678915 0.821121 0.809977 0.815994 1.537243 0.80549 0.812362 0.831126 0.809264 0.792922 0.795165 0.845688 0.832514 0.799295 0.81197 0.833974 0.532863 0.521399 0.855408 0.52414 0.809407 0.518479 0.557075 0.531938 0.378124 0.378124 0.794204 0.81286 35.453 39.102 Cl 0.000423 0.000169 0.001946 0.000113 0.000169 0.000564 0 0.000338 5.64E-05 0.002003 0.001439 0.001185 8.46E-05 5.64E-05 0.000254 0.000987 0.000282 5.64E-05 0.000959 0 0 0.000282 0.000282 0.000903 2.82E-05 0.000649 0.000395 8.46E-05 0.000762 0 0 0.000423 0.000338 0 0.00031 0.000564 0 0.000113 0 0.064028 0.070544 0.00048 5.64E-05 K 0.124137 0.062554 0.022761 0.000716 0.045599 0.106158 0.115211 0.139328 0.128484 0.193826 0.171756 0.182446 0.17549 0.088435 0.000307 0.168457 0.000358 0.000614 0.000153 0 0 0.198788 0.114189 0.126873 0.155286 0.000895 0.1381 0.147179 0.030868 0.007544 0.000742 0.000256 7.67E-05 0.111657 0.000588 0.002455 0.000205 0.000102 0.000256 0 0 0.000307 0.15815 40.08 Ca 0 0.000624 0.000873 7.49E-05 0 0 9.98E-05 0.000175 0 0.000299 9.98E-05 9.98E-05 0.001023 0.001073 0.989746 0 0.000449 0.001073 0.000374 0.000449 0.002645 0.000474 0 0 2.5E-05 0.000374 0 0.000225 0.000225 0.00025 2.5E-05 0.000225 0.000399 0 0 0.000125 9.98E-05 0 0 0 0 0.000449 0.0002 44.956 Sc 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 47.9 Ti V 0.004969 0.00737 0.000835 0.000605 0.000459 0.015282 0.005261 0.007474 0.006326 0.023737 0.0238 0.024635 0.005741 0.001482 0 0.025553 0.00023 0.000376 0.000501 0.000605 0.000271 0.007098 0.007349 0.009937 0.000689 0 0.000919 0 0.000376 0.000459 4.18E-05 0 0.00048 0.002234 0 0 0.000271 0 0 0.000418 0 0 0.004656 50.942 52.996 54.938 55.847 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 Mn 0.000874 0 1.82E-05 0 0 0 0.0004 0 0 0.00213 0.002985 0.003604 0.0004 0.0004 0.004787 0.00435 0 0 0.000382 7.28E-05 0.000146 0 0 0.000601 0 0 0 7.28E-05 0 0 0 7.28E-05 0.000309 0.000291 0.000218 0 0.000291 0.000291 0 0 3.64E-05 0.000237 5.46E-05 Fe 0.021666 0.020341 0.003277 0 0.002686 0.022508 0.014844 0.016599 0.009275 0.116658 0.126632 0.146794 0.032374 0.007932 0.00043 0.108224 0.001397 0.001486 0.001021 0.000376 0.002149 0.022777 0.051408 0.045571 0.016868 0.000376 0.036761 0.018783 0.000537 0.000466 0.000537 0.012821 0.001092 0.017208 0.00231 0 0.000609 0.000985 0.013609 0 0.000752 0.001665 0.00428 Cr 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 63.546 Cu 65.37 Zn 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 Optimum site occupancy 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 mica mica clay qz stilb? mica mica mica mica mica mica mica mica mica apatite mica clay clay clay qz clay mica mica mica mica clay mica mica clay clay clay topaz topaz mica topaz clay topaz topaz topaz zun zun clay mica T + O = 6 -7 T + O = 6 -7 Si = 4 6.074 6.137 4 4 T+O T+O T+O T+O T+O T+O T+O T+O T+O = 6 -7 = 6 -7 = 6 -7 = 6 -7 = 6 -7 = 6 -7 = 6 -7 = 6 -7 = 6 -7 6.107 6.088 6.07 6.084 6.56 6.66 6.66 6.061 6.088 T + O = 6 -7 Si = 4 Si = 4 Si = 4 6.65 4 4 4 4 4 6.036 6.168 6.138 6.108 4 6.098 6.091 4 4 4 3 3 6.064 3 4 3 3 3 18 18 4 6.042 Si = 4 T + O = 6 -7 T + O = 6 -7 T + O = 6 -7 T + O = 6 -7 Si = 4 T + O = 6 -7 T + O = 6 -7 Si = 4 Si = 4 Si = 4 T+O =3 T+O =3 T + O = 6 -7 T+O =3 Si = 4 T+O =3 T+O =3 T+O =3 T + O = 18 T + O = 18 Si = 4 T + O = 6 -7 Charge balance Actual Cation Sum Normalization factor Sum of (-) charge Sum of (+) charge (no Fe) 1.57525247 3.855889844 23.51327947 23.33718118 1.675810813 3.66210789 23.40543653 23.24812209 0.784056113 5.101675673 28 27.40900777 1.576126523 2.537867323 35.96576365 17.98268118 1.647307679 0 24 #DIV/0! 1.635867922 3.733186474 23.52810806 23.35242769 1.610200959 3.78089453 23.42245732 23.30627349 1.557085282 3.898309278 23.56891226 23.43379005 1.599727401 3.80314796 23.58518433 23.51306434 1.584998517 4.138805135 22.16607825 21.14769806 1.580008343 4.215167616 22.49421509 21.37131155 1.563956372 4.258430811 22.57103116 21.25681303 1.465017345 4.137152382 23.57378158 23.29292232 1.601759116 3.800821197 23.5418241 23.47921567 0.007131701 0 24 #DIV/0! 1.603987217 4.145918327 22.47686701 21.54035507 0.821120843 4.871390166 28 27.33431719 0.809976501 4.938414875 28 27.12409393 0.815993734 4.901998429 28 27.75239357 1.540409539 2.596712043 24 17.9925629 0.80549028 4.965919639 28 27.56379289 1.551836776 3.889584326 23.77546192 23.58961468 1.580489571 3.902588231 23.5503915 23.13291827 1.553515548 3.951038667 23.62850185 23.25212033 1.558979283 3.917948153 23.89130226 23.75342122 0.795164851 5.030403439 28 27.78488698 1.592797108 3.828485103 23.60239935 23.30818277 1.588176271 3.835216601 23.73368376 23.58347245 0.799295022 5.004409996 28 27.19131411 0.811970377 4.926288095 28 27.34605805 0.833974222 4.796311318 28 27.16846766 1.611042554 1.862148205 21.62677789 10.73576169 1.608147965 1.865499982 21.85181159 10.88819769 1.579713445 3.83867088 23.31421997 23.17724241 1.614986589 1.857600564 21.68442443 10.80496243 0.809406822 4.941890644 28 27.49714484 1.611971206 1.861075426 21.83700677 10.88196232 1.608754743 1.864796367 21.78730526 10.92710831 1.67745262 1.788426072 21.83106976 10.79357377 1.481169481 12.15255933 13.70581102 55.52216373 1.508463941 11.9326684 14.7113908 55.93134386 0.794203518 5.036492424 28 27.55317198 1.559247924 3.874945034 23.63527963 23.60086211 Hypothetical 10.811 Fe3+ 0.009012265 0.008330444 0.016717221 0 #DIV/0! 0.007627824 0.003935659 0.005706743 0.001568999 0.052730624 0.055355943 0.063994631 0.012985558 0.002309257 #DIV/0! 0.039134095 0.006803739 0.007339489 0.005003204 0.000976435 0.010670409 0.008664895 0.016222277 0.016276463 0.005708956 0.001891569 0.01273754 0.006134012 0.002688279 0.002293471 0.002576492 0.023874122 0.00203763 0.004867955 0.004290839 0 0.001133034 0.001836514 0.024337992 0 0 0.00838709 0.00125148 Fe2+ 0.074530749 0.066161553 0 0 #DIV/0! 0.076398451 0.052188422 0.059000988 0.033706498 0.430094155 0.478417857 0.561117117 0.120951296 0.027840326 #DIV/0! 0.40955483 0 0 0 0 0 0.079926278 0.184403196 0.163776063 0.060377086 0 0.128001979 0.065904633 0 0 0 0 0 0.061186851 0 0 0 0 0 0 0.00897402 0 0.015331539 Fe2+/Fe tot 0.892124256 0.888169949 0 #DIV/0! #DIV/0! 0.909220967 0.929875754 0.911807396 0.955521572 0.890787246 0.896293256 0.897626895 0.903047163 0.923406661 #DIV/0! 0.912781233 0 0 0 0 0 0.902192343 0.919141487 0.909601585 0.913613289 0 0.90949564 0.914851091 0 0 0 0 0 0.926304307 0 #DIV/0! 0 0 0 #DIV/0! 1 0 0.924532458 B 6.939 Li Calculated 18.015 H2 O 4.1035748 4.1880893 14.005302 7.0377233 #DIV/0! 4.2563415 4.0767845 4.1231948 4.2456339 2.3570752 2.6649762 2.7191428 3.8904583 4.1968536 #DIV/0! 2.6906439 14.335262 14.138425 14.478131 6.9376195 14.246343 4.3716077 4.0973002 4.1360935 4.4731201 14.237975 4.2377874 4.3845185 14.091842 14.277265 14.988012 3.9344886 4.4707031 3.888434 4.0838847 14.490091 4.4455045 4.3165979 4.6111386 -2.3326324 -1.9960811 13.784086 4.225193 Atomic parts per formula unit H Li 1.7566397 1.7027183 7.9323354 1.9828818 #DIV/0! 1.764054 1.7112287 1.7844561 1.7925922 1.0830391 1.2471075 1.2855156 1.7868908 1.770912 #DIV/0! 1.2384335 7.7527231 7.751475 7.8791867 2 7.8541434 1.887731 1.7751957 1.8142509 1.9456511 7.9514581 1.8011997 1.8668419 7.8291819 7.8083731 7.9808127 0.8133889 0.9259058 1.65711 0.8422122 7.9498691 0.9185034 0.8936526 0.9155349 -3.1470945 -2.6443046 7.7072934 1.8176398 B 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 F 0.241729 0.296662 0.057736 0.016832 0 0.23384 0.288771 0.214224 0.207193 0.908672 0.746829 0.70944 0.212759 0.228874 0 0.757474 0.245903 0.248246 0.116112 0 0.145857 0.111172 0.223703 0.182183 0.054238 0.045278 0.197288 0.132834 0.167007 0.191627 0.019187 1.185823 1.073463 0.34289 1.157211 0.047343 1.081497 1.106137 1.084465 4.368985 3.802525 0.290292 0.182142 Na 0.01174 0.005734 0.004216 0.001656 0 0.011529 0.00921 0.017635 0.010422 0.031325 0.012834 0.015559 0.035271 0.002976 0 0.007394 0 0 0.000213 0 0.001728 0.021317 0.00662 0.009624 0.012611 0.00372 0.009825 0.018017 0.004789 0.000214 0.005007 0.00162 0 0.011855 0.001293 0.001505 0.001214 0.000487 0 0.074004 0.093427 0.000657 0.01854 Mg 0.137031 0.114629 0.019935 0.000522 0 0.12315 0.213678 0.155054 0.101054 1.937128 1.828445 1.737563 0.236535 0.23419 0 1.910444 0.002404 0.000609 0.00121 0 0.002655 0.125909 0.268873 0.181203 0.048507 0.003104 0.14456 0.11705 0.027377 0.006687 0 0.001072 0.006215 0.20526 0 0.001423 0 0 0.000736 0.004999 0.002454 0 0.093558 Al 2.590886 2.714754 3.740888 0.00301 0 2.66506 2.547641 2.623868 2.771384 1.188169 1.4363 1.455777 2.4956 2.398932 0 1.352164 3.772971 3.700608 3.910589 0.005486 3.841217 2.63414 2.426281 2.537673 2.884081 3.922231 2.571479 2.708759 3.655842 3.761265 3.719621 1.982647 2.017605 2.499361 2.02166 3.826476 2.032891 1.95879 2.023595 13.39475 13.47611 3.848021 2.763817 Si 3.240013 3.206137 4 3.994932 0 3.177714 3.249152 3.197235 3.152228 2.844821 2.748579 2.721296 3.169519 3.417572 0 2.814725 4 4 4 3.991777 4 3.159751 3.243542 3.197435 3.106626 4 3.237705 3.192873 4 4 4 0.992271 0.972669 3.283631 0.973643 4 0.964928 1.038831 0.951331 4.595178 4.512032 4 3.14979 Cl 0.001631 0.00062 0.009929 0.000286 0 0.002106 0 0.001319 0.000215 0.008289 0.006064 0.005045 0.00035 0.000214 0 0.004093 0.001374 0.000279 0.004701 0 0 0.001097 0.001101 0.003566 0.000111 0.003263 0.001512 0.000325 0.003811 0 0 0.000788 0.000631 0 0.000576 0.002788 0 0.00021 0 0.778109 0.841779 0.002415 0.000219 K 0.478658 0.229081 0.116119 0.001817 0 0.396309 0.435603 0.543143 0.488645 0.80221 0.72398 0.776933 0.726028 0.336127 0 0.698408 0.001744 0.003031 0.000752 0 0 0.773202 0.445631 0.501281 0.608403 0.004503 0.528715 0.564464 0.154476 0.037166 0.003557 0.000476 0.000143 0.428613 0.001093 0.012133 0.000381 0.000191 0.000457 0 0 0.001546 0.612824 Ca 0 0.002284 0.004455 0.00019 0 0 0.000377 0.000681 0 0.001239 0.000421 0.000425 0.004232 0.004078 0 0 0.002188 0.005298 0.001835 0.001166 0.013133 0.001844 0 0 9.78E-05 0.001883 0 0.000861 0.001124 0.001229 0.00012 0.000418 0.000745 0 0 0.000617 0.000186 0 0 0 0 0.002262 0.000773 Sc 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 Ti V 0.019159 0.026988 0.00426 0.001536 0 0.05705 0.019891 0.029136 0.024057 0.098243 0.100319 0.104905 0.023752 0.005634 0 0.105942 0.001119 0.001856 0.002456 0.001572 0.001348 0.027609 0.028679 0.039263 0.002699 0 0.003517 0 0.001881 0.002263 0.0002 0 0.000896 0.008575 0 0 0.000505 0 0 0.005074 0 0 0.01804 Cr 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 Mn 0.003369 0 9.29E-05 0 0 0 0.001514 0 0 0.008814 0.012583 0.015348 0.001657 0.001522 0 0.018036 0 0 0.001874 0.000189 0.000723 0 0 0.002373 0 0 0 0.000279 0 0 0 0.000136 0.000577 0.001118 0.000406 0 0.000542 0.000543 0 0 0.000434 0.001192 0.000212 Fe Cu 0.083543 0.074492 0.016717 0 0 0.084026 0.056124 0.064708 0.035275 0.482825 0.533774 0.625112 0.133937 0.03015 0 0.448689 0.006804 0.007339 0.005003 0.000976 0.01067 0.088591 0.200625 0.180053 0.066086 0.001892 0.14074 0.072039 0.002688 0.002293 0.002576 0.023874 0.002038 0.066055 0.004291 0 0.001133 0.001837 0.024338 0 0.008974 0.008387 0.016583 Zn 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 IV Si B 3.240013 3.206137 4 3.994932 0 3.177714 3.249152 3.197235 3.152228 2.844821 2.748579 2.721296 3.169519 3.417572 0 2.814725 4 4 4 3.991777 4 3.159751 3.243542 3.197435 3.106626 4 3.237705 3.192873 4 4 4 0.992271 0.972669 3.283631 0.973643 4 0.964928 1.038831 0.951331 4.595178 4.512032 4 3.14979 Oct 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 Al Sum 0.759987 4 0.793863 4 0 4 0.005068 4 4 4 0.822286 4 0.750848 4 0.802765 4 0.847772 4 1.155179 4 1.251421 4 1.278704 4 0.830481 4 0.582428 4 4 4 1.185275 4 0 4 0 4 0 4 0.008223 4 4.44E-16 4 0.840249 4 0.756458 4 0.802565 4 0.893374 4 0 4 0.762295 4 0.807127 4 0 4 0 4 0 4 3.007729 4 3.027331 4 0.716369 4 3.026357 4 0 4 3.035072 4 2.961169 4 3.048669 4 0 4.595178 0 4.512032 4.44E-16 4 0.85021 4 Al 1.830899 1.920891 3.740888 -0.002058 -4 1.842774 1.796792 1.821103 1.923613 0.03299 0.184879 0.177073 1.665119 1.816504 -4 0.166889 3.772971 3.700608 3.910589 -0.002738 3.841217 1.793891 1.669823 1.735108 1.990708 3.922231 1.809183 1.901632 3.655842 3.761265 3.719621 -1.025082 -1.009726 1.782993 -1.004697 3.826476 -1.00218 -1.00238 -1.025074 13.39475 13.47611 3.848021 1.913607 Mg Fe 2+ Fe 3+ 0.137031 0.0745 0.00901 0.114629 0.0662 0.00833 0.019935 0 0.01672 0.000522 0 0 0 #DIV/0! #DIV/0! 0.12315 0.0764 0.00763 0.213678 0.0522 0.00394 0.155054 0.059 0.00571 0.101054 0.0337 0.00157 1.937128 0.4301 0.05273 1.828445 0.4784 0.05536 1.737563 0.5611 0.06399 0.236535 0.121 0.01299 0.23419 0.0278 0.00231 0 #DIV/0! #DIV/0! 1.910444 0.4096 0.03913 0.002404 0 0.0068 0.000609 0 0.00734 0.00121 0 0.005 0 0 0.00098 0.002655 0 0.01067 0.125909 0.0799 0.00866 0.268873 0.1844 0.01622 0.181203 0.1638 0.01628 0.048507 0.0604 0.00571 0.003104 0 0.00189 0.14456 0.128 0.01274 0.11705 0.0659 0.00613 0.027377 0 0.00269 0.006687 0 0.00229 0 0 0.00258 0.001072 0 0.02387 0.006215 0 0.00204 0.20526 0.0612 0.00487 0 0 0.00429 0.001423 0 0 0 0 0.00113 0 0 0.00184 0.000736 0 0.02434 0.004999 0 0 0.002454 0.009 0 0 0 0.00839 0.093558 0.0153 0.00125 X Mn 0.003369 0 9.29E-05 0 0 0 0.001514 0 0 0.008814 0.012583 0.015348 0.001657 0.001522 0 0.018036 0 0 0.001874 0.000189 0.000723 0 0 0.002373 0 0 0 0.000279 0 0 0 0.000136 0.000577 0.001118 0.000406 0 0.000542 0.000543 0 0 0.000434 0.001192 0.000212 Ti 0.019159 0.026988 0.00426 0.001536 0 0.05705 0.019891 0.029136 0.024057 0.098243 0.100319 0.104905 0.023752 0.005634 0 0.105942 0.001119 0.001856 0.002456 0.001572 0.001348 0.027609 0.028679 0.039263 0.002699 0 0.003517 0 0.001881 0.002263 0.0002 0 0.000896 0.008575 0 0 0.000505 0 0 0.005074 0 0 0.01804 [] 0.926 0.863 0.218106 3 #DIV/0! 0.893 0.912 0.93 0.916 0.44 0.34 0.34 0.939 0.912 #DIV/0! 0.35 0.216702 0.289587 0.078868 3 0.143387 0.964 0.832 0.862 0.892 0.072774 0.902 0.909 0.312212 0.227492 0.277602 4 4 0.936 4 0.172101 4 4 4 0 0 0.142401 0.958 Sum 3 3 4 3 #DIV/0! 3 3 3 3 3 3 3 3 3 #DIV/0! 3 4 4 4 3 4 3 3 3 3 4 3 3 4 4 4 3 3 3 3 4 3 3 3 13.40482 13.48797 4 3 Na 0.01174 0.005734 0.004216 0.001656 0 0.011529 0.00921 0.017635 0.010422 0.031325 0.012834 0.015559 0.035271 0.002976 0 0.007394 0 0 0.000213 0 0.001728 0.021317 0.00662 0.009624 0.012611 0.00372 0.009825 0.018017 0.004789 0.000214 0.005007 0.00162 0 0.011855 0.001293 0.001505 0.001214 0.000487 0 0.074004 0.093427 0.000657 0.01854 K Ca [] Sum 0.48 0 0.509601 0.23 0 0.762901 0.12 0 0 0 0.996337 0 0 1 0.4 0 0.592162 0.44 0 0.55481 0.54 0 0.438541 0.49 0 0.500933 0.8 0 0.165227 0.72 0 0.262765 0.78 0 0.207082 0.73 0 0.234469 0.34 0 0.656819 0 0 1 0.7 0 0.294198 0 0 0.996068 0 0.01 0.991671 0 0 0.9972 0 0 0.998834 0 0.01 0.985139 0.77 0 0.203637 0.45 0 0.547749 0.5 0 0.489095 0.61 0 0.378888 0 0 0.989895 0.53 0 0.46146 0.56 0 0.416658 0.15 0 0.839611 0.04 0 0.961391 0 0 0.991316 0 0 0.997486 0 0 0.999112 0.43 0 0.559532 0 0 0.997615 0.01 0 0.985746 0 0 0.998219 0 0 0.999323 0 0 0.999543 0 0 0.925996 0 0 0.906573 0 0 0.995535 0.61 0 0.367862 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 OH F 0.241729 0.296662 0.057736 0.016832 0 0.23384 0.288771 0.214224 0.207193 0.908672 0.746829 0.70944 0.212759 0.228874 0 0.757474 0.245903 0.248246 0.116112 0 0.145857 0.111172 0.223703 0.182183 0.054238 0.045278 0.197288 0.132834 0.167007 0.191627 0.019187 1.185823 1.073463 0.34289 1.157211 0.047343 1.081497 1.106137 1.084465 4.368985 3.802525 0.290292 0.182142 Oxide weight percent Cl 0.001631 0.00062 0.009929 0.000286 0 0.002106 0 0.001319 0.000215 0.008289 0.006064 0.005045 0.00035 0.000214 0 0.004093 0.001374 0.000279 0.004701 0 0 0.001097 0.001101 0.003566 0.000111 0.003263 0.001512 0.000325 0.003811 0 0 0.000788 0.000631 0 0.000576 0.002788 0 0.00021 0 0.778109 0.841779 0.002415 0.000219 OH Sum 1.75664 1.702718 7.932335 1.982882 2 1.764054 1.711229 1.784456 1.792592 1.083039 1.247108 1.285516 1.786891 1.770912 2 1.238434 7.752723 7.751475 7.879187 2 7.854143 1.887731 1.775196 1.814251 1.945651 7.951458 1.8012 1.866842 7.829182 7.808373 7.980813 0.813389 0.925906 1.65711 0.842212 7.949869 0.918503 0.893653 0.915535 -3.14709 -2.6443 7.707293 1.81764 2 2 8 2 2 2 2 2 2 2 2 2 2 2 2 2 8 8 8 2 8 2 2 2 2 8 2 2 8 8 8 2 2 2 2 8 2 2 2 2 2 8 2 SiO2 50.48884 52.60467 47.1108 94.58314 90.56971 51.14562 51.63554 49.2801 49.80211 41.3003 39.1802 38.39719 46.03256 54.02734 0.072738 40.79327 49.33787 48.66825 49.0298 92.36677 48.39869 48.81158 49.93903 48.62546 47.6435 47.77828 50.81402 50.02246 48.02644 48.78805 50.11018 32.01763 31.32875 51.39807 31.49348 48.63402 31.15333 33.47239 31.962 22.71998 22.71998 47.72051 48.84154 TiO2 0.396998 0.588825 0.066722 0.048374 0.036697 1.221019 0.420351 0.597165 0.505422 1.896582 1.901587 1.968309 0.458716 0.118432 0 2.041704 0.018349 0.030025 0.040033 0.048374 0.021685 0.56714 0.587157 0.793996 0.055046 0 0.073395 0 0.030025 0.036697 0.003336 0 0.038365 0.178482 0 0 0.021685 0 0 0.033361 0 0 0.371977 Al2O3 34.25631 37.79342 37.3834 0.060463 6.615076 36.3952 34.35267 34.31488 37.15099 14.63593 17.3719 17.42858 30.75321 32.17788 0.0359 16.62744 39.48639 38.20343 40.6711 0.1077 39.43538 34.5265 31.69606 32.74472 37.52889 39.75092 34.24308 36.00786 37.24358 38.92522 39.53741 54.28104 55.13886 33.19442 55.48464 39.47506 55.6887 53.5517 57.68588 56.19319 57.57629 38.95167 36.36308 Fe2O3 0.186624 0.181633 0.261643 #DIV/0! #DIV/0! 0.163147 0.083115 0.116888 0.032941 1.017294 1.048596 1.199921 0.250621 0.048512 #DIV/0! 0.753691 0.11152 0.118669 0.081495 0.030025 0.171569 0.177877 0.331908 0.328933 0.116347 0.030025 0.265655 0.127707 0.042892 0.037173 0.042892 1.023698 0.087214 0.101257 0.184437 #DIV/0! 0.048611 0.078636 1.086607 #DIV/0! 0 0.132966 0.025788 FeO 1.388735 1.298025 0 #DIV/0! #DIV/0! 1.470326 0.991718 1.087406 0.636765 7.466158 8.154572 9.467004 2.100476 0.526266 #DIV/0! 7.097411 0 0 0 0 0 1.476369 3.394879 2.978158 1.10719 0 2.40214 1.234624 0 0 0 0 0 1.145212 0 #DIV/0! 0 0 0 #DIV/0! 0.054033 0 0.284269 MgO 1.432608 1.261823 0.157521 0.008291 0.285195 1.329805 2.278245 1.603394 1.07114 18.86765 17.48644 16.44846 2.304775 2.483851 0 18.57582 0.019897 0.004974 0.009949 0 0.021555 1.304934 2.777336 1.848794 0.499091 0.024872 1.522146 1.230319 0.220529 0.054718 0 0.023214 0.134307 2.155545 0 0.011607 0 0 0.016581 0.016581 0.008291 0 0.973311 MnO 0.061979 0 0.001291 0 0 0 0.028407 0 0 0.151075 0.211763 0.255665 0.028407 0.028407 0.339595 0.308606 0 0 0.027116 0.005165 0.01033 0 0 0.042611 0 0 0 0.005165 0 0 0 0.005165 0.021951 0.02066 0.015495 0 0.02066 0.02066 0 0 0.002582 0.016786 0.003874 CaO 0 0.03498 0.048972 0.004198 0 0 0.005597 0.009794 0 0.01679 0.005597 0.005597 0.057367 0.060166 55.50493 0 0.025186 0.060166 0.020988 0.025186 0.148315 0.026585 0 0 0.001399 0.020988 0 0.012593 0.012593 0.013992 0.001399 0.012593 0.022387 0 0 0.006996 0.005597 0 0 0 0 0.025186 0.011194 Na2O 0.094358 0.048527 0.025612 0.02022 0.022916 0.095706 0.075487 0.14019 0.084923 0.234548 0.094358 0.11323 0.264204 0.024264 0.188717 0.055267 0 0 0.001348 0 0.010784 0.169845 0.052571 0.075487 0.09975 0.022916 0.079531 0.145582 0.029656 0.001348 0.032351 0.02696 0 0.095706 0.021568 0.009436 0.02022 0.008088 0 0.188717 0.242636 0.004044 0.148278 K2O 5.847095 2.946435 1.072088 0.033729 2.14779 5.000266 5.426692 6.562623 6.051876 9.129611 8.090047 8.593567 8.265918 4.165483 0.014455 7.934655 0.016864 0.02891 0.007228 0 0 9.363302 5.378508 5.975986 7.314289 0.042161 6.504803 6.932433 1.453944 0.355355 0.034933 0.012046 0.003614 5.259254 0.027706 0.115641 0.009637 0.004818 0.012046 0 0 0.014455 7.449204 H2O F Cl 4.103575 1.191 4.188089 1.539 14.0053 0.215 7.037723 0.126 #DIV/0! 0.083 4.256342 1.19 4.076784 1.451 4.123195 1.044 4.245634 1.035 2.357075 4.171 2.664976 3.366 2.719143 3.165 3.890458 0.977 4.196854 1.144 #DIV/0! 5.382 2.690644 3.471 14.33526 0.959 14.13843 0.955 14.47813 0.45 6.937619 0 14.24634 0.558 4.371608 0.543 4.0973 1.089 4.136093 0.876 4.47312 0.263 14.23798 0.171 4.237787 0.979 4.384519 0.658 14.09184 0.634 14.27727 0.739 14.98801 0.076 3.934489 12.098 4.470703 10.932 3.888434 1.697 4.083885 11.835 14.49009 0.182 4.445505 11.04 4.316598 11.269 4.611139 11.52 -2.33263 6.83 -1.99608 6.054 13.78409 1.095 4.225193 0.893 0.015 0.006 0.069 0.004 0.006 0.02 0 0.012 0.002 0.071 0.051 0.042 0.003 0.002 0.009 0.035 0.01 0.002 0.034 0 0 0.01 0.01 0.032 0.001 0.023 0.014 0.003 0.027 0 0 0.015 0.012 0 0.011 0.02 0 0.004 0 2.27 2.501 0.017 0.002 Subtotal 99.46312 102.4914 100.4173 #DIV/0! #DIV/0! 102.2874 100.8256 98.89164 100.6188 101.315 99.62703 99.80367 95.38671 99.00345 #DIV/0! 100.3845 104.3203 102.2099 104.8512 99.52084 103.0226 101.3487 99.35374 98.45824 99.10262 102.1021 101.1356 100.7643 101.8125 103.2288 104.8265 103.4498 102.1902 99.13404 103.1572 #DIV/0! 102.4539 102.7259 106.8943 #DIV/0! 87.16273 101.7617 99.5927 O=F+Cl -0.50491 -0.64942 -0.10611 -0.05396 -0.0363 -0.50562 -0.61101 -0.44233 -0.43629 -1.77242 -1.42892 -1.34225 -0.41209 -0.48219 -2.26837 -1.46953 -0.40609 -0.4026 -0.19717 0 -0.23497 -0.23091 -0.46083 -0.3761 -0.11097 -0.0772 -0.41541 -0.27776 -0.27307 -0.31119 -0.032 -5.09782 -4.60614 -0.7146 -4.98616 -0.08115 -4.64891 -4.74624 -4.85104 -3.38832 -3.11367 -0.46494 -0.37649 Total 98.95821 101.842 100.3112 #DIV/0! #DIV/0! 101.7818 100.2146 98.44931 100.1825 99.54259 98.19811 98.46142 94.97462 98.52126 #DIV/0! 98.91498 103.9142 101.8073 104.654 99.52084 102.7877 101.1178 98.89291 98.08214 98.99164 102.0249 100.7201 100.4865 101.5394 102.9176 104.7945 98.35201 97.58402 98.41943 98.17105 #DIV/0! 97.80503 97.97964 102.0432 #DIV/0! 84.04906 101.2968 99.21621 Table 1. Representative Electron Microprobe Analyses of Sheet Silicate Minerals Sample no. 1 4-1667 #1 2 3-1917 #14 Ser - Py - Qz Mineral assemblage Ser - Qz - Cp illite3 Mineral 6D-1862 #25 3-1647 #29 Qz - Cl - Py - Bn - Cc illite4 muscovite5 3-1917 #10 3-1917 #16 Qz - Ser Bio - Qz sericite6 biotite (phlogopiteannite series)7 Py - Bio - Qz - Cp Anh biotite (phlogopiteannite series)8 4-1667 #3 17C-1683 #47 Dk - Qz - Py Qz - Hall - Dk - Tp - Py - Cc - Bn kaolinite group clay9 kaolinite group clay10 SiO2 50.49 54.03 47.64 48.84 41.30 40.79 47.11 TiO2 0.40 0.12 0.06 0.37 1.90 2.04 0.07 0.00 Al2O3 34.26 32.18 37.53 36.36 14.64 16.63 37.38 38.95 47.72 Fe2O3* 11 0.19 0.05 0.12 0.03 1.02 0.75 0.26 0.13 FeO* 11 1.39 0.53 1.11 0.28 7.47 7.10 0.00 0.00 MgO 1.43 2.48 0.50 0.97 18.87 18.58 0.16 0.00 MnO 0.06 0.03 0.00 0.00 0.15 0.31 0.00 0.02 CaO 0.00 0.06 0.00 0.01 0.02 0.00 0.05 0.03 Na2O 0.09 0.02 0.10 0.15 0.23 0.06 0.03 0.00 K2O 5.85 4.17 7.31 7.45 9.13 7.93 1.07 0.01 H2O* 4.10 4.20 4.47 4.23 2.36 2.69 14.01 13.78 F 1.19 1.14 0.26 0.89 4.17 3.47 0.22 1.10 Cl 0.02 0.00 0.00 0.00 0.07 0.04 0.07 0.02 99.46 99.00 99.10 99.59 101.32 100.38 100.42 101.76 Subtotal O=(F+Cl) -0.50 -0.48 -0.11 -0.38 -1.77 -1.47 -0.11 -0.46 Total 98.96 98.52 98.99 99.22 99.54 98.91 100.31 101.30 3.24 3.42 3.11 3.15 2.84 2.81 4.00 4.00 AlIV 0.76 0.58 0.89 0.85 1.16 1.19 0.00 0.00 Σ IV 4.00 4.00 4.00 4.00 4.00 4.00 4.00 4.00 Number of moles on basis of Σ IV + VI cations is >6 and <711 Si IV Al VI VI A 1.83 1.82 1.99 1.91 0.03 0.17 3.74 3.85 Mg 0.14 0.23 0.05 0.09 1.94 1.91 0.02 0.00 Fe2+ 0.07 0.03 0.06 0.02 0.43 0.41 0.00 0.00 Fe3+ 0.01 0.00 0.01 0.00 0.05 0.04 0.02 0.01 Mn 0.00 0.00 0.00 0.00 0.01 0.02 0.00 0.00 Ti 0.02 0.01 0.00 0.02 0.10 0.11 0.00 0.00 Σ VI 2.07 2.09 2.11 2.04 2.56 2.65 3.78 3.86 [] 0.93 0.91 0.89 0.96 0.44 0.35 0.22 0.14 Total 3.00 3.00 3.00 3.00 3.00 3.00 4.00 4.00 Na 0.01 0.00 0.01 0.02 0.03 0.01 0.00 0.00 K 0.48 0.34 0.61 0.61 0.80 0.70 0.12 0.00 Ca 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 ΣA 0.49 0.34 0.62 0.63 0.83 0.71 0.12 0.00 [] 0.51 0.66 0.38 0.37 0.17 0.29 0.88 1.00 Total 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 F 0.24 0.23 0.05 0.18 0.91 0.76 0.06 0.29 Cl 0.00 0.00 0.00 0.00 0.01 0.00 0.01 0.00 OH* 1.76 1.77 1.95 1.82 1.08 1.24 7.93 7.71 Analyses by A. Winant; oxides are reported in weight percent 1 Key to sample numbers: drill hole number-footage from collar of hole-# grain number 2 Abbreviations: anh = anhydrite; bio = biotite; bn = bornite; cc = chalcocite; cl = clay; cp = chalcopyrite; dk = dickite; hall = halloysite; py = pyrite; qz = quartz; ser = sericite; tp = topaz 3 Alteration envelope: fine-grained sericite intermixed with clay and fine-grained quartz and pyrite 4 Alteration envelope 5 Vein, with bornite-chalcocite replacing chalcopyrite 6 Alteration envelope: fine-grained sericite 7 Alteration envelope 8 Vein cutting quartz-chacopyrite-sericite vein 9 Clay alteration envelope near quartz veinlet 10 Thick chalcocite-bornite vein, within vein filling jadjacent to chalcocite-bornite 11 Assume hydroxyl site is filled with OH, F, or Cl; optimized to charge balance = 0 and FeIII/(FeII + FeIII) = 0.1 Table 2. Representative Electron Microprobe Analyses of Topaz and Zunyite 1 Sample no. Mineral assemblage2 Mineral 3-1746B #36 3-1746B (1746.5) #40 13-1928 #45 13-1928 #46 Tp-Zun-Cl-Qz-Py-Cc-Bn Tp-Zun-Cl-Qz-Py-Cc Tp-Zun-Py-Cc Tp-Zun-Py-Cc 3 4 topaz 5 topaz zunyite6 zunyite SiO2 32.02 31.49 22.72 TiO2 0.00 0.00 0.03 0.00 Al2O3 54.28 55.48 56.19 57.58 22.72 Fe2O3* 1.02 0.18 0.00 0.00 FeO* 0.00 0.00 0.00 0.01 MgO 0.02 0.00 0.02 0.01 MnO 0.01 0.02 0.00 0.00 CaO 0.01 0.00 0.00 0.00 Na2O 0.03 0.02 0.19 0.24 K2O 0.01 0.03 0.00 0.00 H2O* 3.94 4.09 10.33 10.91 F 12.10 11.84 6.83 6.05 Cl 0.02 0.01 2.27 2.50 100.03 Subtotal 103.46 103.16 98.58 O=F -5.10 -4.99 -3.39 -3.11 Total 98.36 98.18 95.19 96.91 Number of moles on basis of Basis of Σ cations = 18 Σ IV + VI cations = 3 IV VI X Si 0.99 0.97 4.58 4.49 Al 0.01 0.03 0.42 0.51 Σ IV 1.00 1.00 5.00 5.00 Al 1.97 1.99 12.92 12.90 Mg 0.00 0.00 0.00 0.00 Fe2+ 0.00 0.00 0.00 0.01 Fe 3+ 0.02 0.00 0.00 0.00 Mn 0.00 0.00 0.00 0.00 Ti 0.00 0.00 0.01 0.00 Σ VI 2.00 2.00 12.93 12.91 Na 0.00 0.00 0.07 0.09 4.35 3.78 F 1.18 1.16 Cl 0.00 0.00 0.77 0.84 OH* 0.81 0.84 13.87 14.38 0.59 0.58 X F-Topaz7 Analyses by A. Winant; oxides are reported in weight percent; all Fe in topaz assumed to be FeIII 1 Key to sample numbers: drill hole number-footage from collar of hole-# grain number 2 3 Abbreviations: bn = bornite; cc = chalcocite; cl = clay; py = pyrite; qz = quartz; tp = topaz; zun = zunyite Inclusion in pyrite + bornite 4 Inclusion in chalcocite 5 Rimming chalcocite (after pyrite) 6 Rimming chalcocite (after pyrite) 7 X F-Topaz , mole fraction fluor-topaz in topaz solid solution, F / (F + OH*). Table 3. Mineralogy of Veins and Matrix of Crosscutting Relationships Assemblage of Offsetting Vein (Younger) Assem Silicate Alteration Type1 Silicate Alteration Type1 Potassic or none Potassic or none Sericitic Mineralogy qz - bio qz - moly py > cp of vein2,3 qz - moly 1 qz - ser py qz - ser py - cp qz - ser py - bn cc 4 2 1 5 Transitional advanced argillic-sericitic qz - ser qz - ser - py - clay - qz - ser qz - ser - py - clay - alun - bn - py - alun py - clay bn cc bn - cc 1 Uncertain qz - py bn - cc Advanced argillic cp - bn cc > tp qz - clay qz - clay - qz - clay qz - clay py - alun qz - clay - py - tp ± zun - py - qz - clay - py - bn bn - cc alun - clay py - tp bn ± cc bn py cc qz - clay 1 4 2 6 12 4 38 4 5 2 qz - bio py > cp Sericitic qz - ser - py qz - ser - py - cp 1 2 1 2 7 3 1 qz - ser - py - bn - cc Transitional qz - ser - py - clay advanced argillicsericitic 2 qz - ser - py - clay - bn 1 1 1 1 1 qz - ser - py - clay - alun - bn - cc qz - ser - py - alun - bn cc Uncertain qz - py - bn cc cp - bn - cc > tp Advanced argillic 1 qz - clay py - alun bn - cc 2 alun - clay qz - clay py - tp 1 qz - clay py - tp ± bn ± cc qz - clay zun - py bn qz - clay py qz - clay py - bn - cc qz - clay 1 2 3 3 1 1 1 2 6 1 clay 1 1 1 Based on criteria of Seedorff et al. (2005a) Vein filling and alteration envelope are not necessarily distinct, so vein minerals listed include vein filling and envelope (if present); diagonal boxes (patterned )are offsets between similar veins; tallies below the diagonal may represent reversals (see text) Abbreviations: alun = alunite; bio = biotite; bn = bornite; cc = chalcocite; cl = clay; cp = chalcopyrite;moly = molybdenite; py = pyrite; qz = quartz; ser = sericite; tp = topaz; zun = zunyite clay 1 Table 4. Widths and Orientations of Common Vein Types Grouped by Assemblage Assemblage (abbreviated) 1 Alteration Type of Silicate Minerals2 Widths3 (mm) Orientations sericite-chalcocitebornite Sericitic 0.5 - 5 // 0.25 - 6 At variable angles to core axis (0-90 deg). pyrophyllite-clay Advanced argillic 10 - 40 // 5 - 50 alunite-clay Advanced argillic 0.1 - 6 // 0 - 2 Frequently at low angles to core axis (0-30 deg). clay-topaz-zunyite Advanced argillic 5 - 50 // 2 - 35 Frequently at low angles to core axis (0-30 deg). clay Advanced argillic 2 - >100 // 1 - 40 Frequently at low angles to core axis (0-30 deg). 1 2 3 Comments Very rare (two occurrences logged) See Table 5 for definitions of assemblages and their abbreviations Based on criteria of Seedorff et al. (2005a); see also Table 5 Widths of zones are shown where distinct, with width of vein filling followed by two slashes and width of alteration envelope Table 5. Mineral Assemblages Produced by Acidic Fluids at Resolution Alteration Type of Silicate Minerals1 Sericitic Assemblage Intermediate argillic Relative Abundance3 Confidence of Interpretation4 quartz + sericite + pyrite ± chalcopyrite sericite chalcopyrite intermediate low 5 quartz + sericite + pyrite ± chalcocite ± bornite sericite chalcocite bornite high common 5 sericite - clay high very rare 3 topaz - zunyite sericite high very rare 2 quartz + pyrophyllite + kaolinite ± dickite + pyrite pyrophyllite - clay high ± topaz ± bornite ± chalcocite very rare 4 topaz + alunite ± kaolinite ± dickite ± quartz + pyrite ± chalcocite ± bornite topaz - alunite clay7 high rare 4 topaz + quartz ± zunyite ± bornite ± chalcocite topaz - zunyite — rare 3 kaolinite ± dickite ± quartz ± pyrite ± bornite ± chalcocite clay high abundant 5 quartz + alunite ± kaolinite ± dickite ± pyrite ± bornite ± chalcocite6 alunite - clay7 high common 5 quartz + kaolinite ± dickite + pyrite + topaz ± zunyite clay - topaz zunyite — common 5 dickite dickite — low 5 montmorillonite montmorillonite — very common 3 quartz + sericite + pyrite + kaolinite ± dickite ± Transitional chalcocite ± bornite advanced argillic-sericitic quartz + topaz + pyrite ± sericite ± zunyite ± bornite ± chalcocite5 Advanced argillic Abbreviation Sulfidation State of Opaque Minerals2 1 Based on criteria of Seedorff et al. (2005a) Based on criteria of Einaudi et al. (2003); — = not determined by minerals present 3 Relative abundance: very rare, rare, low, common, very common, abundant 4 Confidence in interpretation that these minerals constitute an assemblage ranges from 1 = low confidence to 5 = high confidence 2 5 Characterization of alteration type of this assemblage not definitive; could be transitional advanced argillic-sericitic or sericitic 6 Probably also includes aluminum-phosphate-sulfate (APS) minerals (Harrison, 2007) Though not observed in this study, the assemblage quartz - alunite also is almost certainly present at least locally at Resolution (e.g., Fig. 18 a,b of Troutman, 2001); additional assemblages containing minerals such as andalusite and enargite, also are likely to be documented in the future though it is unlikely that they will prove to be abundant 7