Reductions
advertisement

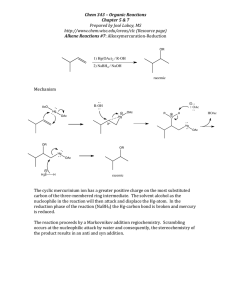
Reductions General Resource: Trost, Comp. Org. Syn. 1991, vol 8 March,1992, chap 19 Carey and Sundberg, vol B, Chap 5 Smith, Organic Synthesis, Chap 4 Material organized (roughly) by transformation From March, 1992, p1208 H H 5% Pd/C Supported metal b/c Pd $$$ and tends to clump. Two general classes: Transition metal hydrogenation and dissolving metal reduction Hydrogenation: Covered in much more detail in Advanced Synthesis and Catalysis. CH3 CH3 H2 5% Pd/C O O no carbonyl reduction M H H H2 5% Pd/C H2 NC General trends: NC Ph more substituted = slower relationship of pressure:rate often not simple many systems pose fire risk! HOAc/HClO4 AcOH HOH EtOH CH2Cl2 EtOAc N N Ph Benzyl survives 10% Pd/C -Same deal as 5%, just more reactive -Often used for hydrogenolysis and more difficult hydrogenations -Mechanism of hydrogenolysis unknown increasing activity Olefin isomerization sometimes a problem MeO N MeO Ph H2 MeO 10% Pd/C MeO NH 90% Note: O2 + H2 Pd/C Fire 5% Pd/ BaSO4 and 5% Pd/CaCO3/Pb(OAc)2 (Lindlar's cat.) and 5% Pd/C/quinoline PtO2 (Adams catalyst) general hydrogenation cat; more active than Pd/C reduced activity H2/PtO2 AcOH/Benzene N quinoline 8 O N N H2 H Raney Nickel (RaNi) -various types available that differ in preparation -sold as 50% wt. dispersion in water -usually wash 5x water, 5x solvent (usually MeOH) -Dry solid is pyrophoric!!! -Remove by filtration under N2 or Ar (pretty good idea for all hydrogenation catalysts) alkyne semi-reduction most common use: H2 5% Pd/CaCO3/Pb(OAc)2 97% Ph Wasserman, TL, 1988, 4977 5% Pd/ BaSO4 Ph 8 Ph O Cl Ph H Ph Ph 87% stereospecific Ni/Al NaOH Ni/H2 + NaAl(OH)4 complementary method: slurry has hydrogenation activity without added H2 Na/NH3 97% stereoselective (more on Na to come) For some applications, can use Ni/Al in 1M NaOH/MeOH Raney Ni Applications OH Ni2B (Nickel boride) Brown, JACS, 1963, 1004, 1005 H2 (275 atm) Ra-Ni OH NaBH4 Ni(OAc)2 Ni2B + H2 (future fuel cell technology??) 86% H2 Ra-Ni vegtable oil in water: More reactive than Ra-Ni Less double bond rearragement margarine in EtOH: Highly selective: Ni2B H2 other uses: O desulfurization Ra-Ni EtOH S 97% TL 1994, 5594 S 61% O Triazene reduction O O H O H HO N N N O Ra-Ni MeOH no added H2 H O H HO H2N 95% Wood, ACIEE, 2004, 1270 Ni2B H2 O 45% Can. J. Chem. 1991, 1554 Diimide Reductions Examples general trends: rate H H N N diimide Unstable -generate in situ -use excess No reaction with -CN, -NO2, Not poisioned by heteroatoms Generation KO2C N N CO2K H2N NH2 (hydrazine) S R R Corey JACS, 2004, 15664 HN KO2CN=NCO2K O II Δ or base NH O Mechanism 88% N HOAc also promotes E-Z isomerization JOC, 1965, 3985 Cu , O2 NH2NH2 CuSO4 5 O2 EtOH/MeOH H N -2 CO2 HO2C as substitution O NH O O JOC, 1977, 3987 Tol O H2N H HOAc rate as strain -HTs R H N N concerted hydrogen transfter ΔGo ~ -50kcal/mol R H HN NH2NH2 O2, Cu(II) NH R Org. Syn. 1969, 30 R N Corey, JACS, 1961, 2957 OAc OAc + N KO2CN=NCO2K CD3OD/CD3CO2D D D syn-exo addition! JACS, 1967, 410 Dissolving Metal Reductions Enones polar solvent M+ M e- + Most common solvents: NH3 (b.p. = -33 oC), MeNH2 (b.p. = -6.3 oC) Li, NH3, 2 equiv EtOH O O +e- Competing process: 2 e- + 2NH3 slow 2NH2- + H2 (rxn mixture is basic) O Birch Reduction review: Rabideau, Marcinov, Org. React, 1992, 42, 1 CN H EtOH vs. CN Li, NH3, 2 equiv EtOH H good overlap EtOH H poor overlap - +e EtOH CN CN EtOH CN +e- +eO explain: OMe OMe Li, NH3, 2 equiv EtOH LiO H Regioselective enolate generation: Li, NH3; H MeI ~50% O Deprotonation here R O Ph Na/NH3 R OH O H H O H Stork, JACS, 1965, 275 Carbonyl Reductions Metal Hydrides-General RCO2H RCH2OH RCO2R RCH2OH RCONR2 RCH2NR2 Ionic Metal Hydrides (LiAlH4, NaBH4, etc) LiAlH4 -very strong reducing agent -flammable -Workup can be trouble b/c Al salts; Feiser workup: for ng LiAlH4, add n mL H2O, n mL 15% NaOH, then 3n mLH2O, filter ppt. -related: Red-Al [NaH2Al(OCH2CH2OMe)2; similar reactivity but greater solubility 2 H M L3 O H 1 2 + M M HL3 O M1 OH Reactivity increases with: -increasing electronegative M1 (Li > Na) -increasing electropositive M2 (Al > B) -increasing e- donation of L (Et > H) -increasing electrophilicity of substrate (RCHO > RCOR) OH MeO2C MeO2C H O O O THF, reflux 72% L H + MHL2 CO2H M O H L O OH Reactivity increases with: -increasing electropositive M (Al > B) -increasing donor ability of substrate (RCO2R > RCOR) H O H H O H OH H LiAlH4 H Neutral Metal Hydrides (i-Bu2AlH, AlH3, B2H6) HO C(CH3)3 H OH Helmchen, JOC, 2000, 5072 OBn O H O CO2Me LiAlH4 O H OBn O OH O H Nicolaou, JACS, 1995, 10252 H O H H 92% O Directed reduction proceeds through intramolecular hydride delivery: H N N LiAlH4 88% H H H Al Ts O O N H O N H H2 Al + H R Overman, JACS, 1999, 700 R LiAlH4 can also reduce alkynes: THPO LiAlH4, Δ 73% OH LiAlH4 120 - 150 oC HO H LiAlH4, Δ 70% Nearby alcohol accelerates OH LiAlH 4 n R Acta. C. Scan. 1073, B27, 2941 90% TMS H H H allylic leaving group leads to allene: forcing conditions are required for unactivated alkynes OH TMS OH n n = 1, 1h, 66 oC, 68% JOC, 1984, 4092 n = 2, 48h, 85 oC, 84% JOC, 1985, 4014 OH OH TL, 1974, 1593 OH LiAlH4 OMe ????? LiBH4 -Seletive reduction of esters and lactones in presence of acids -acids 'protected' as Li salt -solvent effects: ether>thf>iPrOH HO MeO2C CO2H O O BH3-THF H Br HO O H CO2H Br HO LiBH4 O OH Corey, JOC, 1975, 579 CO2H 81% JACS, 1075, 4144 hypothetical example: O O H N O O N O O S HO LiBH4 BH3-THF HO LiBH4 HO O I Williams, JOC, 2004, 1028 Borane complexes (BH3-L) -selective reduction of acids in presence of esters, amides, lactones. Will reduce ketones, aldehydes and olefins -BH3-THF and BH3-Me2S available + ester dibal alcohol O B2H6 O OEt B O + R 3 OH i-Bu2AlH (aka DIBAL or DIBAL-H) -low temp, 1 equiv, ester -> aldehyde -with XS, get alcohol -gives 1,2 reduction of unsaturated esters -commonly: O O OEt O I OH OH O OEt O O N O S O 45% O HO H N HO LiAlH4 OH Ph [O] aldehyde CO2Et O H H OTBS DIBAL, -78 oC 95% O Ph Brown, JACS, 1960,3866 OH O OEt O OTBS H H Nicolaou, Tetrahedron, 1990, 4517 Weinreb's amide to aldehyde S MeO2C H S OHC DIBAL -78 oC H PO NH NH O OP PO H O DIBAL O N OMe Al R R OP OAlR2 R H OMe O JACS, 1982, 6460 O O N OMe stable at low T stable intermediate aminal decomposes to aldehyde on workup PO biotin >70% O OP O PO OP O lactone to lactol Evans, JACS, 1990, 7001 O O 3o amides to aldehydes 3 equiv. DIBAL H Cl NC OTIPS 78% H Cl NC O O OTIPS O O O NMe2 OH LiAlH(OtBu)3 LiAlH(OEt)3 note: nitrile survives 4 steps!! Nitrile to aldehyde xs tBuOH O O O TMSO OTIPS 63% 92% NC DIBAL >71% TMSO O O OTIPS Corey, JACS, 1993, 8871 LiAlH(OtBu)3 LiAlH4 1.5 EtOAc LiAlH(OEt)3 Brown, JACS, 1964, 1089 O Directed Reductions OH general reference for directed rxn: Hoveyda, Evans, Chem Rev. 1993, 1307 many many many ways. Focus here on selectivity issues 2 common modes OH H O R H OH D 99 89 80 3 7 Li/NH3 LiAlH4 NaBH4 LiBH(s-Bu)3 (L-selectride) (i-Bu)2AlH 1 11 20 97 93 O D R R H R' O D M R' H- R' D = donor D M D R O OH OH R' MBH(OAc)3; M usually NMe4 Evans, JACS, 1988, 3560 ax H O O eq disfavored for small H- donors b/c interaction with C2 axial H H eq ax disfavored for large H- donors b/c interaction with C3 axial H H LiAlH4 OH OH H OH O MBH(OAc)3 R -HOAc R' OH O MBH(OAc)3 -HOAc 83 17 O O LiAlH4 ? ? ? 92 8 ? acyclic cases usually follow Felkin-Ahn model or chelate model (if chelating group nearby) to varying degrees. For a chronological presentation, see Smith, Organic Synthesis, chap 4. O OH E Me O H 50:1 O H R' MBH(OAc)3 OH OH OAc B OAc O R OH OH OAc B OAc O 50:1 O HO H OH E Me one isomer OH O O O MBH(OAc)3 OR OH OH OH O OR 6:1 MeOB(Et)2/NaBH4 MeOB(Et)2 NaBH4 OH O R R Tishchenko Reductions: Evans, Hoveyda, JACS, 1990, 6447 O Prasad, TL, 1987, 155 H- Et B Et O -MeOH OH O OH OH R O R H cat. SmI2 O R, R' = alkyl, aryl generally >97:3 OH O O R OEt O OEt R O Sm Ln OEt MeOB(Et)2 NaBH4 OH O CH3CHO OH OH O 5 OEt R R = Me, Aryl OAc OH cat. SmI2 5 96% >99:1 R = Me, Aryl O O O OH O Zn(BH4)2 Oishi, Nakata, Accts. Chem. Res. 1984, 338; Evans JACS 1984, 1154 OH cat. SmI2 L Zn L O O 95% >99:1 BnO OBn OP OH O OP OH O OP CH3CHO OAc OH cat. SmI2 Zn(BH4)2 Me4NBH(OAc)3 OP OH OH OP 19:1 OP OP OH OH OP 4:1 95% >99:1 OP OH directed reduction + monoprotection OH OH O R H- O R likely Sm+3 MeOB(Et)2 NaBH4 98:2 OH O R O R'CHO Enantioselective reductions. For metal-catalyzed, see Advanced Synthesis and Catalysis notes. CBS Reduction: from Corey, Bakshi, and Shibata Reviews: Corey, ACIEE, 1998, p1986; Srebnik, Chem Rev, 1993, 763. H Ph NH H Ph Ph MeB(OH)2 (1.1 equiv) OH N Ph O B (10 mol%) CH3 CBS Catalyst O R BH3-THF (0.6 equiv) R' OH OH RL R = CH3: 97%ee R = Et: 97% ee R = CH2Cl: 95%ee R = (CH2)2CO2Me: 94% ee OH N NO2 MeO RS 95% ee RS 93%ee RL OTBS O O O BF3 R S CH3 94% ee OH MeO H3C Br 99% ee N OH TBDPSO OEt MeO 84% ee O 91% ee 91% ee O OH O Hex ~95% ee MeO OH OH RO HO OR R OH R CH3 note: some data with alternative boranes or R' B-R groups HO H CH3 CH3 SnBu3 94% ee O Bu3Sn CH3 93% ee 95% ee 91% ee OH H3C OH CH3 85% ee R' R OEt R = Ph, R' = n-alk: 70-90 % ee R = Ph, R' = s-alky: 95% ee R = H, R' = alk: ~96% ee R = TIPS, R' = alk: >90% ee Proposed mechanism for CBS reduction Important points: • Borane in catalyst is Lewis acid; Nitrogen is Lewis base to coordinate second borane • Borane coordination forms cis-5,5 system (a-face in 5) • Borane coordination increases Lewis acidity of catalyst (at B) and activates BH3 as hydride donor • Carbonyl coordination trans to bulky or electron rich group • Hydride transfer via 6-membered TS • Disproportionation between 8 and BH3 + (RO)2BH allows <1 equiv BH3 Enantioselective reduction: Alpine borane and Dip-Cl Review: Brown, JOMC, 500, 1995, p1. Background: asymmetric hydroboration of olefins. An improved reagent: DIP-Cl; Review, Brown, JOMC, 1995, v500, p1. Conjugated systems: 1,4 addition -recall Na/NH3 1,2 vs. 1,4 Luche reduction (original report: JACS, 1978, 2226; review Molander, Chem Rev. 1992, 29) O OH H- Selectrides: MBH(s-Bu)3; M = K, Na, Li available -useful for regioselective enolate generation O TIPSO + NaBH4 NaBH4/CeCl3 49 1 51 99 TfO O O O O NaBH4, CeCl3 O O OBn Cl OBn O OPMB N3 O O 75% 1,2 addtion expanded: 2. O OH O Ar-CeIII O H AcO H Wood, unpublished Ph O O 1. CeCl3 O 75%, 1 diastereomer Ph Ph O O Li H L-Selectride; NCS OH NaBH4, CeCl3 Ph H AcO >80% O Overman, JACS, 1993, 9293 OH O O >80% O O OPMB N3 N3 OtBu OtBu -Ce(+3) coordinates to carbonyl; promotes selective 1,2 addition. -Requires stoichiometric quantity of ANHYDROUS CeCl3 N3 TIPSO L-selectride; PhNTf2 OtBu OtBu 75% Heterocycles, 1989, 703 Ionic Reductions Reductive amination -Usually with NaCNBH3 or NaBH(OAc)3 -Usually in presence of acid to promote imium ion formation -Alternative to amine alkylation (often get over alkylation) -reduction of cation (usually from protonation) -need to avoid H- + H+ -CF3CO2H/Et3SiH is most common combination OH OH CF3CO2H Et3SiH O OH OH O + TBSO O 93% Chem Comm. 1986, 1568 O CHO + OAc N H NaBH(OAc)3, Sn(OTf)2, 4A MS TBSO OP O O OAc TMSOTf Et3SiH OAc O + OP O O N H H N OAc OMe O O CO2Me CF3CO2H Et3SiH N OMe O O H OAc TL, 2000, 6435 JACS, 2004, 516 N CO2Me N N N H N H H OH O Nicolaou, JACS, 2004, 613 2 + HN NH NaBH3CN, AcOH 95% H N HN O O BocHN 2 O BocHN N H HN JACS, 2004, 557 α,β-unsaturated ketones give olefin migration: O R R R R O Reduction of Tosylhydrazones Maryanoff, JACS, 1973, 3662 Baker, JOC, 1975, 1834 O R H2N-NHTs R Ts HN DMF/sulfolane AcOH/NaCNBH3 N R H2NNHTs, HCl, NaCNBH3 Ts HN R NNHTs NH R NaCNBH3, AcOH R -TsH (pKa=7.1) ~75% JOC 1978, 2299 -N2 R HN R N R R 2 possible mechanisms: O H2NNHTs, TsOH, NaCNBH3 DMF/sulfolane N Bn Ts HN N Bn H- 79% O O 3 O CN O 3 5 NNHTs H H R O " CN -N2 R H R -N2 N H R How would you distinguish between them? HO Boeckman, JACS, 1989, 2737 N R ZnCl2, NaCNBH3 ~50% H H N 5 75% HO HN N H H Related chemistry: TBS N N SO2Ar Li R'Li R R Wolf-Kishner Brutal conditions TBS N N SO2Ar R' R N TBS N AcOH -N2, -AcOTBS R' 3 O R R' 3 synthesis of unfunctionalized sp -sp bonds EtO2C N2H4, NaOEt 170 oC N H N H Myers, JACS, 1998, 8891 50-58% Org. Syn. 1995, Coll vol 3, 513 OMOM R Li O 1.MeO MeO C4H9 OMe OMe C4H9 NN(TBS)Ts AcO 2. HCl, MeOH 73% R N2H4 NaOH (HOCH2CH2)2O reflux, then HO 210 oC O 69% Barton, J. Chem. Soc., 1955, 2056 C4H9 MeO cylindrocyclophane F MeO Smith, JACS, 1999, 7423 C4H9 OMe HO OMe H2N B N HN N H H -N2 H H Shapiro reaction useful for difficult olefins; usually low yielding with side products review: Shapiro, Org. Rxns. 1976, 405 TsHN 2 equiv RLi N Li TsN Li N N H Other Methods Clemmensen Reduction review: Vedejs, Org. Rxns. 1975, 22, 401 O Cl N Li Cl Zn(Hg) HCl 56% -N2 H generally poor E/Z selectivity in acylic cases TsHN Li BuLi JOC, 1969, 1109 A fine method if low yields of unfunctionalized products are needed. Review: Org. Rxns. 1962, 356 Ph Ph MeO Ph low yield Swenton, JACS, 1971, 4808 N(CHO)Me H SMe SMe H N O H O TsHNN TsHNN Cl Desulfurization See hydrogenation above. Ra-Ni/H2 almost always used N Ph Cl Ra-Ni/H2 N(CHO)Me H H N O H O Woodward, JACS, 1948, 2107 MeLi NNHTs MeO 20% bullvalene TL 1972, 2589 OH Generally useful method, but: -lota tin -3o thiocarbamates can be difficult to make -1o radicals difficult to form common methods we won't cover: -alkyl tosylate + LiAlH4 -conversion to halide/dehalogenation -elimination/hydrogenation S O O S O O Barton deoxygenation N N AIBN, Bu3SnH 140 oC HO O O O CN CN H CN N N AIBN NC Bu3SnH temp 50 70 100 40% t1/2 74h 4.8h 7.2min TL, 1988, 281 Im S S Bu3Sn H O R AIBN, Bu3SnH 90 oC O C10H21 91% O O O C10H21 OH O JOC, 2000, 6035 Bu3SnH S O SnBu3 R AIBN, Bu3SnH O O O PO S O SnBu3 R Barton: Tet, 1983, 2609; 1987, 3541; 1991, 8969 Synthesis, 1988, 417, 489 S 75% JOC, 1989, 5678 O O "NBSH" Myers Diazene method O2 S N H NO2 PPh3/DEAD/ R OH Barton Decarboxylation Barton, Chem. Comm. 1983, 939; Tet, 1987, 2733 NH2 NH2 N SO2Ar R O R O N H R S -HSO2Ar R H -N2 -CO2 R N NH O R N O S Very useful for unhindered alcohols SSnBu3 - N SnBu3 note: thiohydroxamic acid often labile enough that no Sn is needed, just ambient light. Photolysis works too. OH MeO H3C PPh3/DEAD/NBSH N O S N N O O MeO O Cl Cl N OH PPh3/DEAD/NBSH; O2 ; Me2S Eaton, ACIEE, 1992, 1421 O 1. i-BuOCOC 2. S CO2H O H N N O N H H N ONa N O 3. tBuSH, hν O H N H H Martin, JOC, 1995, 3236 65% OH Holy cow! How does this happen? quant. O N Myers, JACS, 1997, 8572 O S AIBN, Bu3SnH O Reductive couplings and related reactions. Reductive cleavage of strained rings: electron transfer-promoted reductions (part II) OSmX2 O SmI2 I from: -CH2CH2 O O SmI2 + Sm(0) PMP O SmI2 HMPA PMP O I -almost always in THF (can do in Me3CCN) -Very air sensitive -reactivity modulated by additives (JACS 2004, 44; JACS 2000, 7718, SYLETT, 1996, 633) -Kagan discovered, Molander exploited, Flowers studied -Rxns usually psycho fast -Reviews: Molander, Chem Rev. 1992, 29; 1996, 307 (example from here). O +2 Sm OSm+3 +2 ROH OH Sm X2Sm from [2+2] O AcO O O SePh OH Sm OH ROH O Guanacastepene A OH Sorensen, JACS, 2006, 7025 O OSm+3 OTBS CO2Me O SmI2, HMPA 5 min TMS TMS HO SmI2 79% Intermolecular additions of ketyl radicals 97% 93:7 OTBS O CO2Me Corey, JACS, 1987, 6187 + CO2Et SmI2 tBuOH O n-C7H15 O >99:1 dr ketyl can be intercepted: S O OCN OH S S SmI2, LiCl S X2SmO X2Sm OCN or radical addition PMP +3 _ O O OH _ Less likely: via SePhBr 50% S Ph S O + H CN SmI2 THF, MeOH O R H OH O N H Wood, ACIEE, 2004, 1270 O CN Ph H3C OH 99:1 dr M O CN Intramolecular couplings: Pinacol Couplings with SmI2 O SmI2, HMPA THF, tBuOH CO2Me O 2 Mn 2 OMn+1 OH 2 CO2Me HO OH 80% H H SmI2, HMPA O O OH O 2 SmI2 -PhS OP H HO H OH 80% poor dr SmI2, HMPA 78% N H Ph HN 2 SmI2 Ph O PO OP OP Ph Ph Ph Ph O n-Hex n-Hex OP OP OP H OH O OH OH O PO With SmI2 (Chem Rev, 1996, 307) H O 86% O PhS NH 93%; 4:1 OP OP OP SmI2 O O O O 54% OP H PO HO OH OH O OH OP 92:8 OMe N HO OP OP OH O OP OH H HO OP 2.5 SmI2 tBuOH, THF OH OH O O SmI2 ~65% N Cbz H2N O Grayanotoxin III Shirahama, JOC, 1994, 5532 Note cleavage of N-OMe Nicolaou, en route to diazonamide JACS, 2004, 12897. N HO OBn NBn NMOM Cr-mediated reductive coupling of Sp2-X with aldehydes: the Nozaki-Hiyama-Kishi (NHK) reaction X + CrCl2 (>2 equiv) Ni(II) cat. DMSO or DMF O OH X = Br, I, OTf Ni(II) 2Cr(II) 2Cr(III) 2Cr(III) X Ni(0) 2Cr(II) Ni(II) NiX General characteristics Reliable for late-stage coupling Broad functional group compatibility (ketones, ester, nitriles) Nuclophile formed in presence of electrophile (Barbier), so intramolecular (cyclizations) possible Often poor diastereoselectivity Catalytic conditions (in Cr) have been developed: Furstner, JACS, 1996, 12349 Enantioselective versions have been developed: Kishi, JACS, 2004, 12248; OL, 2008, 3073. Review: Furstner, Chem Rev. 99, 991 Cr(III) O CrCl2 + OCrCl2 Intermolecular additions: From Chem. Rev. 1999, 991 Intramolecular additions: Allylations and alkynylations: