Gary Galeucia June 1986
advertisement

CONTROLLING ACID DEPOSITION
BY SEASONAL GAS SUBSTITUTION
IN COAL- AND OIL-FIRED POWERPLANTS
Gary Galeucia
MIT Energy Laboratory Report No. MIT-EL 86-004
June 1986
Controlling Acid Deposition By Seasonal Gas Substitution
In Coal-And Oil-Fired Power Plants
by
Gary B.Galeucia
Submitted to the Alfred P. Sloan School of Management on May 16, 1986,in partial
fulfillment of the requirements for the Degreeof Masterof Sciencein Management.
Abstract
Acid deposition, primarily the result of sulfur emissions due to fossil fuel
combustion, is a serious environmental problem. Resolving the problem will impose
costs measuring in the billions of dollars. Basedon evidence that the rate of wet
sulfate depositionin eastern North Americais higher in the summer half of the year
than in the winter half of the year, seasonal control of emissions is proposedas a
means of minimizing acid deposition control costs. This paper evaluates the proposal
that natural gas be substituted for coal and oil in electric power plants during April
through September.
A model is presented that simulates the substitution of natural gas for coal and oil
in power plants in the eastern 31 state region so as to minimize total costs with
respect to deposition reductions at an Adirondack receptor. The results of the model
show: 1) changes in fuel consumption as a result of substitution, 2) the increased
effectiveness of seasonal versus year-round controls, and 3) the costs of achieving
various levels of deposition reduction at an Adirondack receptor.
The costs of seasonal gas substitution, in terms of emission and deposition
reductions, are compared to cost estimates for other proposed control methods and
strategies. An example is given that calculates the cost with respect to deposition of a
source-oriented control strategy, so that the cost of seasonal gas substitution can be
fairly compared with it. The conclusion of these cost comparisons is that seasonal gas
substitution is cost-competitive with some other control methods, at least in some
states.
Thesis Supervisor: Dr. Henry D.Jacoby, Professor of Management
Acknowledgement: This research was performed at the MITEnergy Laboratory with
the support of the Electric Utility Program and under the supervision of Prof. James
A. Fay and Dr. Dan S. Golomb. Their assistance and guidance is gratefully
acknowledged.
Table of Contents
Page
Title Page
I
Abstract
2
I. Introduction
11.Acid Deposition
S
III. The Old And New Of Acid Rain Policy
10
IV. Seasonal Gas Substitution
13
V. The Seasonal Gas Substitution Model
16
VI. Results Of The Model
23
VII. Gas Supply For Substitution
29
VIII. Other Costs
31
IX. Conclusions
32
References
33
Tables
35
Figures
46
Appendix A
61
Appendix B
74
Appendix C
89
I. INTRODUCTION
Until recently, air pollution was considered a local problem. Now it is known that
winds can carry air pollutants hundreds of miles from their points of origin.
Transported air pollutants can damage aquatic ecosystems, crops, manmade materials,
forests, and human health. The process by which air pollutants damage these
resources is referred to as "acid rain". The term acid rain is used to describe the
complexchemical changes that result from the presence of oxidesof sulfur, oxidesof
nitrogen, and other compounds in the air that may lead to increased acidity in
precipitation, in ground and surface waters, and in soil. A more comprehensive and
accurate term is acid deposition, since the transfer of acid material from the
atmosphere to the biosphere may occur not only in the aqueous phase (rain, snow,
fog, etc.) but also as dry deposition, in which gaseous or particulate material is
adsorbed by the ground, vegetation, or surface water.
Precipitation acidity' considerably below pH 5.6 has been observed in the eastern
United States and Canada, as well as many other areas in the world. Increased acidity
in precipitation and dry deposition of acidic material may increase the acidity of
surface waters, with consequent adverse impacts on fish and other aquatic life.
Increased acidity may also affect vegetation, such as forests or crops, directly or
indirectly through changes in the soil.
It has also been claimed that increased acidity of surface water could adversely
impact human health by mobilizing toxic ions such as lead and copper into drinking
water. However, there appears to be little reason to believe that such health effects
*Acidity is usually measured on a logarithmic scale called pH. PH is defined as the negative
logarithm of the hydrogen ion concentration, which is measured in molar equivalents per liter.
A
neutral solution has a pH - 7.0, and the sale ranges from pH - 0 (strong wid) to pH - 14 (strong
alkali).
Carbon dioxide dissolves in water to form a weak acid; the pH for pure water in
equilibrium with C02 is 5.6.
4
will become a significant public policy issue; the main concerns about the effects of
acid deposition seem to be the adverse consequences for aquatic and terrestrial
ecological systems.
Sulfur dioxide (SO2)is the major chemical compound responsible for precipitation
acidity; it is produced largely as the result of the combustion of fossil fuels. i.e. coal
and petroleum products. S02, along with other chemical compounds, is oxidized into
acid compoundsprimarily in the atmosphere. Precipitation and gravity cause these
acid compounds to be deposited on the Earth's surface, sometimes at great distances
from the sources of the original pollutants. The sources of these pollutants include
electric utilities, automobiles, and smeters.
These pollution sources exist as the result of economic activity.
Consequently,
reducing pollutant emissions is not vithout cost. Economictheory tells us that
pollutant emissions should be reduced to the point vhere the marginal cost of
reducing the emissions equals the marginal benefit derived from the lover emission
level. This simple principle is greatly complicated by uncertainties regarding the
magnitude of the costs and benefits of lover emission levels. It is complicated further
because these pollutants cross political boundaries to damage areas far from the
sources of the economic activity that generated the emissions. Consequently, political
realities and questions of equity are part of the problem.
What is known of the acid rain problem is that there are identifiable and
quantifiable sourcesof emissions.and that there are areas suffering varying degrees
of damage due, at least in part to these emissions.
Formulating a policy that balances costs and benefits, let alone political and equity
concerns, is a very complex and continuing task. Acid rain policy has evolved rapidly
in the 1980's. It has moved away from legislation calling for broad-based emissions
reductions toward more efficient policies that recognize the sial
relationshi
between emissions sources and the areas sensitive to the deposition caused by the
emissions.
This paper presents evidence that acid rain policy should step beyond the
recognition of these spatial relationships tovard a recognition of
relationshins between emissions and deposition.
mSoral
What is meant by temporal
relationships is that there are seasonal variations in depositionrates for a relatively
constant rate of emissions. Just as it is more efficient to seek relatively greater control
of emissions from sources that are relatively close to sensitive areas, it is also more
5
efficient to exert relatively greater control of emissionswhen the depositionrate as a
result of the emissionsis highest.
As one means of controlling emissions when deposition rates are highest, this
paper investigates the impacts of substituting natural as for coal and oil in electric
utility boilers during April through September. A seasonal gas substitution model has
been developedto quantify the costs of this strategy for various levels of deposition
reduction. The modelis static in that it is run for a single year, 1983;this means that
actual price and quantity daa for coal, oil, and gas comes from that year. The model is
concerned with emissions of sulfur dioxide (S02). from electric utilities in the 31
eastern states and the District of Columbia (DC).as veil as the resulting deoosition of
sulfate (S04) at a sinaglerecentor in the Adirondack Mountains of NewYork.
The paper starts by describing hov acid deposition is formed as a result of
emissions from fossil fuel combustion. This is followed by a presentation of the
finding that depositionrates are seasonally variable for a relatively constant rate of
emissions. Next. the policy dilemma that acid rain creates is briefly described and is
followed by a review of hov acid rain policy has evolved from source-oriented to
receptor-oriented control strategies. By combining the idea of receptor-oriented or
targeted strategies with the evidence of seasonal variation in deposition rates, a new
type of targeted control strategy is created. The original targeted strategy related
emission sources and deposition receptors spatially. The new targeted strategy. in
addition to being spatially targeted. is targeted temporally in order to take advantage
of seasonal variations in deposition rates.
To utiliz this new strategy, seasonal substitution of natural gas for coal and oil is
proposed. A model is presented that simulates the substitution of natural gas for coal
and oil so as to minimize the cost of achieving deposition reductions. The results of the
model show: 1) the changes in fuel consumption as a result of substitution, 2) the
increased effectiveness of seasonal versus annual gas substitution, and 3) the costs of
seasonal gas substitution. The costs, in terms of emission and deposition reductions
achieved, are compared to cost estimates for other proposed control methods and
strategies. An example is given that calculates the cost with respect to deposition of a
source-oriented strategy, so that the cost of seasonal gas substitution can be fairly
compared with it. The conclusion of these cost comparisons is that seasonal gas
substitution is cost-competitive with these control strategies. at least in some states.
The model does not consider two important factors: 1) the availability of gas supply,
6
and 2) the capital cost for seasonal gas substitution. These factors are discussed
briefly, with the conclusions being that: 1) there may be restrictive limits to gas
supply and deliverability, and 2) capital costs for seasonal gas substitution are
probably very low relative to capital-intensive control methods such as flue gas
desulfurization. The paper ends by restating the conclusions made throughout.
7
II.
ACID DEPOSITION
II. 1. How Acid Deposition is Formed
The dominant precursors of acid deposition are sulfur dioxide (S02) and nitrogen
oxides(NOx). The sulfur oxide orecursors. the focus of this oer.
are primarily
oroduced by burning sulfur-contining fuels (e.g. coal and oil). After release into the
atmosphere, the sulfur oxides (SOx)will oxidize and can form acids when combined
with water. The particular sequence of changes a pollutant undergoes depends on the
physical and chemical characteristics of the air mass in which it travels. These
characteristics (e.g. initial concentrations of pollutants, wind speed, air turbulence.
sunlight intensity, temperature, rainfall frequency) are highly variable, which is
why scientists cannot precisely characterize the detailedpath of a pollutant from its
"source" to its "sink".
To become acid. emitted S02 must be oxidized either: 1) in the gas phase. 2) after
absorptions into water droplets, or 3) after dry deposition on the ground. The
transformed pollutant can be deposited in wet form (as rain, snow, or fog), or in dry
form (due to particles containing the pollutant settling out of the atmosphere). The
amount of time a pollutant remains in the atmosphere. and therefore how far it is
transported, depends significantly on its chemical form. For example, S02 gas is
dry-depositedat a greater rate than sulfate particles (products of oxidation). If S02is
quickly converted to sulfate (SO4),a smaller fraction of emitted sulfur compounds will
be deposited locally. in the absence of precipitation. The rate of conversion from S02
to S04 depends on the chemical composition of the atmosphere. The frequency and
intensity of precipitation controls the rate of wet sulfate deposition.
Dry deposition is believed to occur at a fairly constant rate over time (i.e. a certain
percentage of the S02 in the air is dry-deposited each hour). with some variability
induced by local conditions. Wet deposition is episodic, and the amount deposited
varies considerably even within a rainfall event. For example, a short rain may
deposit heavy doses if pollutants have been forming and accumulating in the local
atmosphere over time. Without sufficient time for pollutant concentrations to
accumulate, a second rainfall event in quick succession may result in little new acid
deposition.
In general, areas close to emission sources receive significant proportions of their
pollution from steady dry deposition of S02. Areas remote from emission sources
receive a greater share of total deposition from wet deposition, since much of the S02
8
available for dry deposition has been depleted or converted to a wet form. Deosition
in this paper refers to wet sulfate (S04) deposition. Air over any particular area will
carry some residual pollution from distant areas, as well as infusions from nearer
sources. The continuous replenishment and depletion of pollutants along the path of
the air mass, makes precise source-receptor relationships difficult to determine.
II. 2. Seasonal Variation In Deposition Rates
Analysis of several years of precipitation chemistry data has established that wet
sulfate deposition rates in the northeastern U.S.and southeastern Canada are higher
in summer months (April-September) than in winter months (October-March)
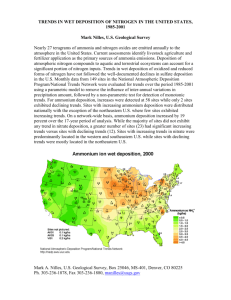
(Boversox et al., 1985;Golomb et al., 1985). Figure 1 shows the seasonal patterns of
sulfate depositionover three years at four receptors. Seasonaldifferences in sulfate
deposition can be clearly seen.
The exact causes of the differences in seasonal deposition patterns are not
perfectly understood; they are probably linked to seasonal storm tracks. Raynor and
Hayes (1982) observed that sulfate (and hydrogen) ion concentrations are highest in
precipitation associated with cold fronts and squall lines, which occur most frequently
in summer months. These higher concentrations are apparently due to the faster
conversion of the emitted sulfur dioxide into sulfate in summer. The quantity of
sulfate being depositedin a stormis a function of the previous trajectory of the warm,
moist air mass and the amount of precipitation in the storm. In winter, more of the
unoxidized S02 is blown offshore and hence does not fall on the land as acid wet
sulfate.
Although the chain of processes from emissons of pollutants to eventual deposition
of acid and acid-producing substances is complex and not fully understood, all
evidence points to a relationship between emissions and deposition. Current scientific
understanding suggests that reducing sulfur dioxide emissions would reduce the
deposition of sulfates. The greatest potential for reducing acid deposition in the
eastern U.S.comes from the reduction of S02emissions.
g
III. THE OLD AND NEW OF ACID RAIN POLICY
III. 1. The Policy Dilemma
Fossil fuels are vital to the U.S. economy's production of goods and services.
Hovever, burning these fuels also produceslarge quantities of pollutnts--substances
that. once released into the atmosphere, can damage natural resources, health.
agricultural crops, manmad materials, and visibility. Consequently, our Nation's lays
and oolicies must strike a balance between the economic benefits and the risks of
fosil fuel combustion.
Recognition of the risks of damage has led some individuals and groups to call on
the federal government to control pollutant emissions, most specifically sulfur dioxide,
more stringently than current as require. Others,pointing to uncertainties about
the causes and consequences of transported pollutants, are concerned that more
stringent emission controls may be mandated prematurely or at too great a cost.
Transported air pollutants also raise significant equity issues. The individuals
served by the activities which generate emissions can be different from those vho
incur resource damage. Similarly,particular groups and regions might bear the costs
of controlling emissions, while others receive the benefits.
Transported air pollutants have become an issue for potential federal action
because they cross political boundaries. The current federal system of pollution
control relies on state-level abatement programs to limit pollution levels in individual
states.
(National emission standards for nev sources of pollution--New Source
Performance Standards--are the exception to this.) Hovever. no effective means of
controlling extensive pollution transport across state lines currently exists.
Transported pollutants also cross the international boundary into and from Canada.
Article 1, Section 10of the Constitution prohibits states from entering into agreements
vith foreign nations without the consent of Congress:thus. any pollution control
agreements vith CaLnadwould require federal action.
Existing federal air pollution control mechanism are governed primarily by the
Clean Air Act. To date, control strategies developed under the Act have ocused on
controlling local ambient air concentrations. The effectiveness of this approach for
controlling transported air pollutants is questionable. For example, the so called "tall
stacks" approach has been used by utiditis to meet local ambient standards as specified
10
by the Clean Air Act. By releasing emissions far enough above the ground. the
pollutants are carried away from the local area, and Clean Air Act compliance is
attained. The pollutants are transported away from the local area, but are not reduced
in total. For any acid rain policy to be effective it must specifically control emissions
that can be transported through the atmosphere to receptors with resources sensitive
to acidity.
III. 2. Source-Oriented Control Strategies
A dynamic linkage exists between acid rain policy formulation and the control
strategies that will be called for when policy is formulated. To illustrate, in the first
years of this decade the emphasis of policy was on controlling S02 emissions. The
early theory was simply that emissions caused acid rain. Therefore, most legislative
proposals of the early 1980'scalled for broad-based emission reductions and distributed
the reductions proportionally throughout the eastern 31 states. These proposals are
known as source-oriented control strateies
because they are concerned only with
emissions at the source and do not consider source proximity to adversely impacted
areas.
The emphasis of recent policy has evolved as more has been learned about acid
rain. What has been learned is that: "First, ...in the northeastern U.S.and southeastern
Canada the rainfall is more acidic than rainfall elsewhere in the country; secondly,
this same region is located close to those areas in the U.S.and Canada which have the
greatest density of sulfur oxides emissions. Thirdly, there are acidified clear lakes
-lakes not directly affected by man's activities- in areas that receive heavy acid
deposition, and in contrast there are few affected lakes where deposition is light. Most
scientists active in the field believe that acidic deposition has been a major
contributor to the acidification of these lakes. But not all areas in the eastern US. are
sensitive to acid rain. The areas at risk are those which receive the depositionand
have limited buffering capacity" (Eltins, 1985).
Notice that Mr. Elkins', who is Director, Office of Program Development, Office of
Air and Radiation, U.S. EPA.emphasis is on acid deoosition rather than emissions. the
effects of deposition, and the sensitivity to acid deosition. Control strategies that are
concerned with the proximity of emission sources to adversely impacted areas are
known as targeted or receptor-oriented strateies. Mr. Elkins is telling us something
about the direction of acid rain policy, namely that when EPAis ready to make an acid
11
rain control policy recommendation, targeted control strategies are likely to be part of
that policy.
This emphasis on acid deposition and targeted control strategies is manifesting
iself in EPA'sresearch agenda. "Weare now greatly expanding our research efforts to
deal with the gaps in our knowledge,and to put our country in a better position to
recommend targeted and efficient policies" (Elkins. 1985). EPA's research mission is
explicitly directed at economically efficient, targeted control policy, with particular
attention tovard deposition and sensitivity to deposition. The task at had is to identify
emission control methods that mesh with this olic orientation.
1II1.3. Targeted Control Strategies
Recent work in atmospheric modeling has brought new moaning to the idea of
targeted strategy.
The traditional definition says that source/receptor
pollutant
transport relationships exist that make it more efficient to identify areas sensitive to
deposition and then use those transport relationships to identify the primary sources
that contribute to deposition in the sensitive area.
This definition could be
characterized a being spatially targeted.
The nev, added dimension to the idea of targeted strategy can be characterized as
being temporally targeted. Differences in seasonal rates of sulfate desition create
the ogoortunity for seasonal control of sulfur emissionsas a more effective means of
reducing annual amounts of sulfate deoosition. By encouraging or requiring S02
emissionsto be curtailed in the summer half of the year, there is a larger reduction of
annual depositionper ton of S02 removed than if the same quantity were removed
year-round.
Therefore, it may prove to be less expensive to reduce deposition by
controlling emissionsonly in the summer half of the year, rather than year-round.
In other words,there will be a larger reduction in annual depositionper dollar spent
controlling emissionsduring the summerhalf of the year. than if the samenumber of
dollars were spent controlling emissions year-round.
12
IV. SEASONAL GAS SUBSTITUTION
IV. 1. Why Natural Gas?
Seasonal control of emissions can be accomplished by substituting lower sulfur
fuels for higher sulfur fuels during periods with higher deposition rates (i.e.
April-September). This naer evaluates the annual vet sulfate deoosition reduction
that would result from substituting natural gas for coal and residual oil in utility
boilers during April through SeDtember.
Natural gas was chosen as a substitute fuel because it produces virtually no sulfur
dioxide when burned. Seasonal gas substitution allows a continued utilization of
existing coal resources in the winter half (October -March) of the year and increased
utilization of natural gas during the summer half (April-September) of the year.
While the fuel price differential between gas and coal may be substantial, the capital
required for retrofit gas burner installation is expected to be quite low. Thus. the
comparative annual cost to a achieve a given target deposition reduction -- by seasonal
fuel switching to natural gas vs. year-round scrubber operation-- may very well turn
out to be in favor of gas subsitution. This is precisely the goal of the aper: estimating
the costs of seasonal gas substitution in sulfur emitting power plants in absolute units
as well as relative to the costs that would result if these plants installed emission
control devices (e.g. scrubbers) to achieve the same amount of sulfate deposition at an
environmentally sensitive receptor.
Imaortant factors to be considered in seasonal natural gas substitution strategies
include:
1. In the summer months there is currently excess capacity in the natural gas
distribution system. According to Wilkinson (1984) only 78% of the pipeline capacity
is used in the summer months. and in some regions as little as 51%. Summer gas
supply and deliverability will be discussed later in this paper.
2. Seasonal gas substitution could be implemented rapidly relative to the period needed
to install scrubbers or develop "clean burning" technology for a large number of
plants. The quick implementation schedule would allay fears that further delays in
reducing acid deposition may cause irreparable damage to the environment.
Anticiated benefits beyond lower sulfate deposition, from seasonal gas substitution
include:
1. Improved local air quality with lower ambient air concentrations of S02 and
13
particulates.
2. Improved visibility.
3. Increasedpotential for achieving attainment in non-attainment areas.
4. Decreased dependence upon imported oil.
5. Reduced sensitivity to fuel supply disruptions e.g. coal strikes or oil embargos.
6. Increased reliance on domestic energy resources.
7. Decreased consumption of limestone and other sulfur-capture materials used in
emission controls.
8. Decreased land requirements and cost for scrubber sludge and flyash disposal.
IV.2. Natural Gasas a Boiler Fuel
Natural gas has never been a favorite utility boiler fuel in most parts of the
eastern U.S. Combustionof natural gas produces more than 10% of total btu output by
electric utilities in only seven of the eastern 31 states (EIA, 1984a). The primary
reason for this pattern is that natural gas is an expensive boiler fuel relative to coal.
This reason is certainly a viable one. There are two less viable reasons why natural
gas may continue to be disfavored as a boiler fuel.
The first concerns the perception by some that gas reserves are imminently
exhaustible.
A reasonable range for the amount of the remaining conventional
natural gas in the U.S. Lower 48 that is recoverable under present and easily
forseeable technological and economic conditions is 430 to 900 trillion cubic feet (TCF)
as of December 1982 (TA, 1985). (This resource estimate does not include Alaskan,
Canadian, Mexican, or unconventional resources.) At a consumption rate of 20 TCFper
year., slightly higher than present consumption, the resource estimated above will last
21 to 4 vears. The best explanation for this misperception of imminent exhaustibility
is that in the 1970'sgas demand exceeded gas supply as a result of price controls on
natural gas. The market disequilibrium created the image that we were running out
sooner rather than later.
This first misperception led policymakers to restrict gas use. which in turn has
created a second misperception, namely that gas use is restricted. Restrictions on gas
use in electric utility power plants were enacted when the federal Powerplant and
Industrial Fuel Use Act (PIFUA) of 1978 was signed into law on November 9, 1978.
However, PIFUA restrictions were sharply repealed by the Omnibus Budget
Reconciliation Act signed into law on August 13, 1981. Since the 1981amendment, the
14
PIFUArestrictions on natural
as use do not aoolv to "eii
n"
ovwerDlnts at all. A
pover plant is "existing"if it vas in service or under construction prior to November
9. 1978 (Bardin., 1985). Furthermore,
exemptions are available to post-1978 powver
plants. Pre-1978 pover plants contribute the bulk of total S02 emissions because a)
most generating units vere built prior to 1978.and b) older plants ae subject to less
restrictive pollution control regultions.
15
V. THE SEASONAL GAS SUBSTITUTION MODEL
V. 1. General Description
The analysis in this paper relies upon a model developed to evaluate the annual wet
sulfate deposition reduction that would result from substituting natural gas for coal
and residual oil in utility boilers during April through September. The model does not
consider load dispatching as a means of reducing emissions, i.e. generating more
power from an existing gas-fired plant or turbine that has excess capacity in summer
and wheeling that electricity, rather than seasonally substituting gas in coal- or
oil-fired plants. The inclusion of load dispatching strategies is left for future analyses.
The seasonal gas substitution model estimates the corresonding
annual control costs
and fuel substitution amounts for any level of deoosition reduction.
The model's S02emission sources are 387 utility plants burning coal or residual oil
as a primary boiler fuel in the eastern 31 states and D.C.The criteria for including a
plant in the modelwere that it had to have a rated capacity of 50 megawattsor larger,
and at least 10%of total btus had to be generated from either coal or oil. The names,
locations, and fuel characteristics of these plants are listed in Appendix A. Refer to
the guideat the beginning of the Appendixfor columndefinitions.
The atmospheric transport model, known as the MIT acid deposition model (Fay et
al.. 1985; Golomb et al.. 1985; Kumar. 1985). is an adaptation of the Fay-Rosenzweig
climatologicallong-range transport modeloriginally developedfor estimating annual
average S02 concentrations in the U.S. (Fay et al., 1980). It is empirically determined
in that the model parameters are derived by comparison with airborne concentrations
and wet deposition measurements.
Because the physical and chemical processes that pollutants undergo is highly
variable, the accuracy of long range atmospheric transport models is frequently
questioned. Even among those scientists that develop them there is considerable
variability in the estimationof the transfer coefficients. In spite of this, the MITacid
deposition model has been well received by those knowledgeable in the field.
Therefore, it is justifiably appropriate to use for this analysis.
The MIT acid deposition model derives transfer coefficients which estimate the
quantity of deposition at a receptor per unit of emission at a source. Transfer
coefficients have been derived for both an annual and a seasonal (summer/winter)
16
basis. The seasonal gas subsitution model uses the summer transfer coefficients to
relate emissions reductions, as a result of substituting natural gas for coal and oil, to
deposition reductions at an Adirondack receptor.
Table 1 lists the values of the
seasonal and annual transfer coefficients between the 31 eastern states plus D.C.and
an Adirondack receptor. Table 1 shows that the summer transfer coefficients are on
average nearly twice as large as the winter ones. In other words, on average. summer
emissions from the 31 eastern states cause nearly twice the deoosition at an
Adirondack receptor as an equal Ouantity of winter emissions.
The transfer coefficient Tij is the ratio of the amount of deposition at receptor j
contributed by source i divided by the emission amount Qifrom source i. The total
deposition Djat receptor j equals the sum of the products of the transfer coefficient Tij
times the emission Qi :
Dj - i Ti
(1)
When seasonal transfer coefficients are used, the annual deposition is obtained by
summing seperately the product of the transfer coefficient and emissions for summer
(April- September) and winter (March-October):
(Dj)an -
i
(Tij Qi)vi + 7 i (Tij Qi)su
(2)
In the seasonal gas substitution model the emission-deposition relationship takes
the functional form,
(Dj)su -'i (Tij Qi)su(3)
where the transfer coefficients (Tij)su are constants, the summer deposition (Di)su is
0 i)su are dependent variables. By
the independent variable, and summer emissions (Q
selecting a desired summer deposition quantity, the required level of emissions is
determined, which in turn determines the amount of gas substitution necessary to
achieve the desired deposition quantity for the April-September period.
The same transfer coefficient is used for all emission sources within a state. This is
valid for states distant from the receptor, but may be questionable for states close to
the Adirondacks. For instance, New York state has emission sources both to the west
and south of the Adirondacks. The higher the variation in direction and range from
the sources within a state to the receptor, the less appropriate it is to use a single
transfer coefficient for all sources within that state. The use of single transfer
17
coefficients within a state was chosen for this analysis because: ) the bulk of
deposition at an Adirondack receptor comes from distant states, and 2) it simplifies the
presentation of the analysis. The use of multiple transfer coefficients within a state is
left for future analysis.
V. 2. Functional
Form
The model is in fact a linear program (LP) which seeks to minimize the
incremental spending on natural gas as a result of substitution. For each electric
power plant i. there is a cost differential between a given btu quantity of gas and coal
and/or gas and oil. Multiplying this cost differential by the quantity of gas substituted
equals the incremental spending on fuel by the power plant.
Minimization of the incremental spending on natural gas is performed subject to
two types of constraints. The first constraint specifies the desired level of deposition
and has already been described above by Eq. (3). The second type of constraint
requires that the same quantity of btus are producedby each power plant under the
gas substitution strategy as were actually produced when no substitution occured. The
btu output of each source is equal to the btu content of the coal, oil, or gas multiplied
by the quantity of coal. oil. or gas consumed. Actual btu output was determined from
fuel heat content and consumption data (EIA,19684).
The LP model in its functional form seeks to minimize the sum of the products:
MIN
i
(4)
Fi Gi
subject to:
Dsu(target) = 2 i (Tij Qi)su
(5)
(btu)i - HCi Ci + H
(6)
i
+ Hg Gi
where the symbols are:
(btu)i - seasonal(April-September)total btu output for power plant i.
Ci - seasonal quantity of coalburned by power plant i,
Dsu- target seasonaldepositionquantity for a specifiedreceptor,
Fi - fuel cost differential between gas and coal and/or gas and oil at power
plant i.
Gi - seasonal quantity
of natural
gas substituted for
18
coal and/or
oil at
power plant i.
HCi- heat content of coal consumed by power plant i,
Hg
heat content of natural gas (one cubic foot- 1000 btu assumed for all
power plants).
H°i- heat content of oil consumed by power plant i.
The Adirondacks receptor is used in the model because it is environmentally
sensitive and centrally located with respect to other environmentally sensitive areas
in the U.S.and Canada. By adding additional deposition constraints. the model could be
made to consider more than one receptor. This would require the use of a unique set of
transfer coefficients for each additional receptor. For simplicity of presentation, the
model has been limited to a single receptor.
However, it is possible to speculate as to the effect of multple receptors.
For
instance, if a Southern Applachian receptor were used in addition to an Adirondack
receptor, more substitution would occur in southern states. Increased substitution in
southern states in order to reduce Southern Applachian deposition would also reduce
Adirondacks deposition by a small amount. As a result. less substitution would be
required in northern states in order to achieve the same depositionreduction in the
Adirondacks. Thus, there is a spillover effect when multiple receptors are used. The
inclusion of multiple receptors is left for future analyses.
V. 3. Emissions
Most legislative proposals to date have focused on a 31 state region encompassing
the states east of, and bordering on, the Mississippi River. Ofthe 26 to 27 million tons
of sulfur dioxide emitted in the continental United States in 1980,about 22 million tons
came from this 31 state region. The model uses 22 million tons as the base level when
calculating percentage reductions in emissions. This paper calculates that the electric
utilities included in this analysis we resoonsible for aOproximatelv 16 million tons of
S02 emissions (Table 2), or 73% of the 22 million ton total (assuming 1983 total
emissions were equal to those in 1980).
Table 2 lists 1983emissions of S02 attributable to the burning of coal and residual
oil in electric power plant boilers in the 31 easternmost states and DC.i.e. the power
plants in Appendix A. Emissions were calculated from annual electric utility coal and
oil consumption data (EIA, 1984a)neglecting any sulfur removal processes which may
19
have been used in that year. These emissions are used by the model for calculating
deposition at an Adirondack receptor,
Since in moststates sulfur emission rates are fairly constant throughout the year
(NAPAP, 1985), the model assumes that fuel consumption during April through
September is equal to one-half of annual fuel consumption. Therefore. emissions
during April through September are assumed to equal to one-half of annual emissions.
To assess this assumption, net generation data (trillion kilowatthours of output) was
compiled for coal-fired plants in the eastern 31 state region (Figure 2). Figure 2 shows
that monthly variations in net generation do occur. However,if the monthly figures
are summed for the periods April-September and October-March, the former period
accounts for 51% of annual net generation. From this, it can be safely inferred that
emissions during April through September are equal to one-half of annual emissions
in the eastern 31 state region. This does not necessarily hold true for individual states:
future analyses may wish to account for state-level variations in seasonal fuel
consumption and S02emissions.
V.4. Deposition
The amount of wet sulfate deposition at a receptor can be linearly related to the
amounts of sulfur emissions from sources using transfer coefficients. These transfer
coefficients, and the MIT acid deposition model from which they were derived, were
discussed earlier. Total annual wet sulfate deposition at an Adirondack receptor was
estimated to be 27.5 kilograms sulfate per hectare per year (kg S04 haly - l)(Fay et al.,
1985). This figure is used as the base for calculating percentage reductions in total
annual wet sulfate deposition at an Adirondack receptor. Table 3 contains the summer
and annual deposition amounts. at an Adirondack receptor, which were calculated to
have been contributed by the sources included in this analysis. (Note: It is necessary
to multiply the figures in Table 3 by a factor of three in order to convert sulfur (S) to
sulfate (S04). S04 is three times the molecular weight of S.) This aPer calculates that
electric utilities in the eastern 31 states contribute 142 k S04 h
1 total.
-
annually to an
Adirondack receptor. or 52% of the 275 k S04 ha
Of this 14.2kg annual total,
11.2 kg or 79% is calculated to be deposited between April and October. Summer
deposition is disproportionately higher because the summer transfer coefficients are
nearly twice as large on average as winter ones (see Table 1).
20
V. 5. Calculating the Cost of Seasonal Gas Substitution
Incremental
spending on natural gas by utilities is assumed to equal the
incremental quantity of natural gas consumed at a power plant as a result of
substitution, multiplied by the cost differential between gas and coal, or gas and oil, at
that plant, summed for all such power plants. It should be noted that the costs derived
here for seasonal gas substitution are solely the result of the price differentials
between gas and coal or oil. Preliminary estimates of the incremental capital and
operating costs associated with seasonal gas substitution indicate that the fuel price
differential is by far the major cost. Because capital and operating costs for seasonal
gas substitutionare uncertain and relatively small, this paper will leave the inclusion
of these factors to future analyses.
The coal and oil prices used in the analysis are actual average prices per million
btu paid by the power plants in 1983 (EIA, 1984a). These prices are listed in Appendix
A. columns 5 and 10. The gas prices used are the state-average cost per million btu
paid by electric utilities in that state (Table4). If no electric utility burned gas in a
state, then the average price paid by industrial consumers was used (EIA, 1984b). From
the gas prices listed in Table 4, it can be seen that prices vary significantly from state
to state. Using the data in Appendix A and Table 4, the plant-level price differentials
have been calculated, and are shown in Appendix B.
The actual coal and oil prices, as well as the state-average gas prices, are not
necessarily indicative of present and future prices, and therefore of price
differentials, for these fuels. A fall in oil prices, which are determined in the world
market, could be expected to produce a decrease in natural gas prices because the two
fuels are to some extent substitutes. Coal prices are affected to a greater extent by
production costs, and to a lesser extent by the prices of oil and gas because these fuels
are not closesubstitutes. Hence, a fall in oil prices and a subsequentfall in gas prices
should be accompanied by a relatively smaller decrease in coal prices. The result is
that in a period of lower oil prices, a smaller price differential between gas and coal
could be expected.
To test this hypothesis informally, it is useful to look at gas. coal, and oil prices and
price differentials over time (Figure 3). (Prices have been taken from EIA, 1985and
are adjusted to 1983 dollars using the U.S. Bureau of Labor Statistics producer price
index for crude energy materials.) During the period 1983to 1985,the price of oil rose
fairly steadily throughout 1983 and into mid-1984, and then declined during the
21
remainder of 1984and throughout 1985. The price of gas followed a similar pattern to
that of oil, but the rise and fall are less pronounced. The price of coal remained
relatively stable throughout the period. So, the hypothesis is substantiated, at least
during this short period.
The implicationsof this for fuel price differentials are shown in Figures 4a and b.
Figure 4a showsthat the gas/coal price differential rose and fell with the samepattern
as the price of gas itself. Thus, the direction of the price of gas reveals the direction of
the gas/coal price differential. In the current environment of lover as prices.
seasonal sa substitution for coal is eausllr. if not more. econoaical than it was in
19B3.
In regard to the gas/oil price differential, Figure 4b shows that seasonal gas
substitution for oil becomesmore attractive when the oil price is rising and less
attractive when it falls. In the current environment of lover oil prices, the gas/oil
price differential is smaller, but it isstill negative. Hence, there is still an economic
incentive for gas substitution.
The historic prices are used here as a first approximationand illustration of the
fuel price differential trends in current years. More detailed explanations and
forecastsof fuel prices are left to future analyses.
V. 6. Using the Seasonal Gas Substitution Model
The model, described earlier, is a linear program which seeks to minimize the
incremental spending on natural gas subject to constraints on deposition and btu
output. The model is exercised by selecting various target levels of deposition
reduction and then solving the model. The modelselects a oover
lant to use seasonal
m substitution based on: 1) the rate at vhich it contributes to deosition. and 2) the
fuel orice differential it faces. The rate at which a plant contributes to depositionis a
function of the sulfur content of the fuel per million btu and the transfer coefficient
between the power plant and the receptor. Power plants where these two factors are
relatively lrge will be selected first for gas substitution. Similarly. power plants that
have smaller fuel price differentials will be selected first.
Beyond the cost of seasonal gas substitution, the model shows which plants switch,
how much gas consumption increases, and how much coal and oil consumption
decrease; from which the effect on emissions can be calculated.
22
VI.
RESULTS OFTHE MODEL
Appendix C is a sequential list of the 387 plants as they enter the solution.
Plant-level and cumulative data are also provided. To use the Appendix, look in column
14 for the desired percentage reduction in deposition. This and all preceding plants
have been selected for gas substitution. Reading horizontally, columns 5 and 7
indicate the cumulative amount of coal and oil displaced, column 10 indicates the
amount of gas substituted, column 11 indicates the sulfur emission reduction, and
column 17 indicates the total cost. Refer to the guide at the beginning of the Appendix
for definitions of all the columns.
Each time the model was exercised, total annual deposition was reduced in 5%
increments.
Corresponding levels of gas substitution, coal and oil displacement,
emission reductions, and resultant cost were calculated for each 5% decrement. These
results are summarized in Table 5. For example. in the case of a 20% sulfate deposition
reduction, 909 billion cubic feet (bcf) of natural gas are substituted for 53 million tons
of coal and 87 million barrels of oil at a cost of $2.929billion (1983$). with a resulting
emission reduction of 2.9 million tons of S02. For a 30% sulfate deposition reduction,
these quantities increase to 1440bcf of gas being substituted for 97 million tons of coal
and 94 million barrels of oil at a cost of $5.858 billion, with a resulting emission
reduction of 4.8 million tons of SO2.
From this data, the total, average, and marginal cost curves for seasonal gas
substitution with respect to deposition can be derived (Figures 5. 6, and 7). Total cost
starts at negative $0.204billion for a 5% deposition reduction, and rises nonlinearly to
$10.931 billion for a 40% deoosition reduction. Cost is initially negative because a
negative price differential exists between gas and oil at some plants. Because its
objective is to minimize cost, the seasonal gas substitution model chooses the plants
with negative price differentials first. This condition raises the question of why these
plants do not convert from oil to gas regardless of pollution concerns. Some oil-fired
plants have converted since 1983,e.g. Boston Edison's Mystic #7 burns gas seasonally.
The others have not for reasons that the model fails to consider. Perhaps gas supply is
unavailable or insufficient, or perhaps utility management has no incentive to
convert given its monopoly power.
Total, average, and marginal cost curves are useful for comparing the costs of
various control methods and strategies. They will be used later in the paper when
23
seasonal gas substitution is compared with another proposed control strategy.
VI. 1. The Effectiveness of Seasonal Control Strategies
For evaluating the effectiveness of emission reduction schemes, Golomb et al.
(1985) defined a gain factor (GF) as the ratio of the fractional deposition decrement at
a chosen receptor (here, Adirondacks). to the fractional emission decrement in the
eastern 31 state region that occurs as a result of the reduction scheme. Dividing the
fractional deposition decrement at an Adirondack receptor by the corresponding
fractional emission reduction at the various levels of gas substitution produces a series
of gain factors for this strategy. For each level of percentage decrease in deposition,
there is a corresponding percentage reduction in emissions as a result of seasonal gas
substitution. The GF is calculated from the model results and is a measure of the
overall effectiveness of any deposition control strategy.
To show the increased effectiveness of seasonal over year-round controls, a
comparison of the gain factors from these two strategies is made. Using Eq.(2), it is
possible to calculate the annual deposition reduction that would result at each level of
gas substitution if the same quantity of gas were substituted year-round instead of
during April through September. When a given quantity of gas is substituted, the S02
emission reduction remains the same regardless of whether substitution occured
seasonally or year-round.
Substituting a given quantity of gas for coal or oil will
reduce emissions by some constant amount regardless of when during the year
substitution occurs; the same is not true with respect to deposition. The annual
deposition reduction is smaller in the case of year-round controls because the winter
transfer coefficients are smaller. Table 6 presents the GFfor each of several levels of
seasonal and year-round gas substitution. For example, for a 25% reduction in
deposition, there is a corresponding 3.9 million ton or 18%(if a 22 million ton base is
assumed) reduction in S02emissions, (due to 1176bcf of natural gas being substituted
for coal and oil). If this same quantity of gas were evenly substituted year-round,
emissions would still be reduced by 18%, but, because both summer and winter
transfer coefficients are used, the deposition reduction is now 16%. The GF for
seasonal substitution is (25%/18%)-1.40, while that of year-round substitution is
(16%/18%)-0.88. Hence, seasonal substitution is 59% [(1.40%/0.88%)-1.591 more
effective than year-round substitution at this level. Figure 8 is a graphical
comparison of the gain factors for seasonal and year-round controls. The
24
effectiveness varies somewhat because the relative amount of gas substitution
occuring in each state varies at different levels of deposition reduction.
The GFsfor both seasonal and annual controls are diminished at higher levels of
deposition reduction. This is because plants further from the receptor are included in
the solution as the deposition reduction becomes larger. The distance between the two
lines in Figure 6 represents the superiority of seasonalover annual gas substitution.
This distance remains relatively stable regardless of the level of deposition reduction.
In general. a seasonal control strategy is 52-60%more effective than an annual
control strategy for reducing depositionat an Adirondackrecentor.
VI.2. The Effect of Substitution on Fuel Consumption
Each plant in the model burns a known quantity of coal or oil annually (EIA,
1984a).It has been assumedthat half this quantity is consumedduring April through
September. This is supported by evidence that net generation by coal-fired plants
during April through September is 51%of annual net generation in the eastern 31
state region during 1983 (Figure 2).
When a plant is chosen by the model for gas substitution, the coal or oil it would
burn during April through September is replaced by natural gas. The quantity of gas
substituted is determined by calculating the quantity of gas that would be needed to
replacethe btu output that the coalor oil wouldproduce. The average heat content for
coal and oil at each plant is used for this calculation (EIA. 1984a).
For natural gas, a heat content of 1000btu per cubic foot is assumed. Acutal average
heat content for natural gas in the U.S. is approximately 1050 btu/cubic foot (EIA,
1984b);a 5% reduction to 1000 btu/cubic foot has been allowed to account for boiler
derating. i.e. a 5% loss in boiler output. Basically, derating occurs because a boiler
designed to burn coal or oil does not burn gas with equal effectiveness because of
differences in the combustioncharacteristics of the fuels. Experience with derating
due to gas substitution is meager, since gas substitution in coal and oil burners is very
limited at present. Five percent is a reasonable allowance. based on experience with
substituting natural gas for oil at Boston Edison's Mystic 7 unit (Boston Edison, 1985).
Detailed work on dersting and inefficiencies caused by burning gas in boilers
designed for coal or oil is left to future analyses.
The model calculates the quantities of gas substituted and coal and oil displaced for
the various levels of deposition reduction that the model was exercised for, as shown in
25
the bottom half of Table 5. These quantities were mentioned earlier in the case of 20%
and 30%deposition reductions. Figures 9, 10,and 11show curves of these quantities.
Initially most of the gas is substituted for oil. However, because oil's contribution
to deposition is very small, coal quickly becomes the object of substitution. To
illustrate, Figure 11 shows that for a 5% deposition reduction, gas displaces 79 million
out of a possible 97 million barrels of oil, i.e. 80%. For coal. Figure 10 shows that 8
million out of a possible 176 million tons, i.e. less than 5%, is displaced. For a 20%
deposition reduction, the respective percentages for oil and coal are 90%and 30%,oil's
percentage rises only slightly while coal's percentage increases by a factor of six.
Hence, beyond the lowest levels of deposition reduction, substituting gas for coal is
nearlv,comnletelv resoQnsible for further reductions.
VI. 3. The Average Cost of Emission Reduction
Figure 12 shows the reductions in S02 emissions for various reductions in
deposition. Using this and the total cost data, the average cost of emission reductions at
various levels of deposition reduction can be calculated (Figure 13). The average cost
of reducing S02 emissions via seasonal gas substitution ranes
from a negative $340
nor ton S02 at the 5% denosition reduction level (from Figure 12 this corresponds to a
0.6 million ton reduction in emissions). to a ositive S1584 oer ton S02 at the 40%
deposition reduciton level (a corresponding 6.9 million ton emission reduction). (The
cost of emission reduction is negative when there is a negative price differential, i.e.
when oil is more expensive per btu than natural gas). For comparison, the S02
removal cost by limestone flue gas desulfurization (scrubber) was reported to be in the
range $576-1126per ton (Miller, 1985). From the sixth column in Table 5, it can be
seen that the average cost of seasonal gas substitution is in or below the average cost
range for scrubbing, for up to a 25% reduction in total annual deposition at the
Adirondack receptor. The conclusion is that there are a substantial number of plants
where the cost of seasonal gas substitution is competitive with that of scrubbing.
As noted earlier, the costs of seasonal gas substitution are based on 1983 fuel price
differentials. Future price differentials may vary, consequently, future costs may be
different,
VI. 4. The Average Costof DepositionReduction
The debate over alternative strategies for controlling acid rain has focused mainly
26
on the expected costs of reducing S02 emissions. Total cost and S/ton of S02 removed
are frequently used to compare alternative control strategies. In making these
comparisons, a distinction should be made between receptor-oriented (or targeted)
strategies that maximize the amount of deposition reduction at a receptor(s) for a unit
of emission reduction, and source-oriented strategies aimed solely at reducing total
emissions. A direct comparison of the cost of receptor- and source-oriented strategies
can be misleading; these strategies will not result in equal deposition reductions at a
given receptor for equal emission reductions.
In order to facilitate a direct comparison, it is useful to define a cost per unit of
deposition reduction at a particular receptor. Dividing the total cost for seasonal gas
substitution by the corresponding quantity of deposition reduction at a receptor,
produces a measure of the average cost of reducing deposition at that receptor. The
average cost of deposition reduction at an Adirondack receptor, expressed in terms of
billions of dollars per kg S04 per hectare per year (B$/kg S04 ha I yrl), is shown in
Table 5, column 4. The average cost for deposition reduction ranges from a negative
$0.165 billion/ kt S04 hair-lt.
for a 5% deposition reduction to $1.002 billion/ kt S04
hI;!-i
for a 40%'deoosition reduction.
After first determining the resulting deposition reduction, the average cost of
deposition reduction may be calculated for any source-oriented emission control
strategy. Cost comparisons, based on the cost of deposition reduction rather than the
cost of emission reduction, can then be made between seasonal gas substitution and
source-oriented control strategies.
The following section illustrates such a
comparison.
Morrison and Rubin (1985) developed a model that computes the emission
reduction and cost that would result from emission caps of 1.5 and 1.2 lbs. S02 per
million btu on utility emissions using optimized combinations of switching to lower
sulfur coal and flue gas desulfurization (FGD). The 1.5 and 1.2 lbs. emission caps
resulted in annual emission reductions of 8 and 10million tons S02respectively. Based
on the distribution of emission reductions across the eastern 31 states, these emission
reductions would respectively yield 7.2 and 8.2 kg S04 ha 1 yfrt deposition reductions
at an Adirondack receptor (calculated using the MIT acid deposition model annual
transfer coefficients). These quantities of deposition reduction are respectively
equivalent to 26% and 30% reductions from the 27.5 kg S04 hal 1 base deposition level
at an Adirondack receptor.
27
Table 7 summarizes the following comparisons. Morrison and Rubin calculated
total cost ranges of $1.5-2.6and 3.2-4.7 billion (1980S)for the S and 10 million tons of
S02 emission reductions, respectively. Using a GNP deflator of 1.2 to adjust to 1983
dollars makes the cost ranges $1.8-3.1 and 3.8-5.6 billion, respectively. Dividing the
cost by the deposition reductions gives $0.25-0.43 and 0.46-0.68 billion per kg S04
deposition removed, respectively, for the two cases. Referring to Table 5, column 4,
the average cost of deposition reduction for similar (25% and 30%) reductions via
seasonal gas substitution is $0.64and 0.72 billion per kg. The conclusion is that, for
25% and 30% deposition reductions fat an Adirondack receptor, Morrison and Rubin's
optimized straegy has a lower average cost per kilogram of S04 reduced than does
seasonal gas substitution. This is not to my that seasonal gas substitution is not
cost-competitive with other control strategies, in this case an optimized combination
of switching to lower sulfur coal and FGD. I respectfully submit that Morrison and
Rubin's is but one control strategy; other control strategies will have different costs,
some higher and some lover. The purpose of the preceding comparison is primarily to
show that cost comparisons with respect to deposition can be made between sourceand receptor-oriented strategies.
Using the data in Appendix C, state-level average costs were computed for 25% and
30% deposition reductions (Table 8). Figures 14 and 15 show this data ranked from
lowest to highest, plotted against the cumulative percentage deposition reduction of
the states. For example, in Figure 14.Ohio's (OH) average cost is $0.724 billion per kg
S04 hal, at the 25% deposition reduction level; it accounts for an approximate 5%
deposition reduction by itself, and together with preceding states accounts for a 12%
deposition reduction.
While the average costs for seasonal gas substitution in the entire eastern 31 state
region are higher than those derived from Morrison and Rubin's two cases for
equivalent deposition reductions, there are several states that do have average costs of
achieving deposition reduction via seasonal gas substitution that are within the
ranges of Morrison and Rubin's cases. The following states, DC,FL,MA.NJ, NY,RI, and
VA, have average costs that are in or below the ranges specified by Morrison and
Rubin, namely $0.25-0.43 billion (25% reduction) and $0.46-0.68 billion (30%
reduction) per kg S04 ha' 1 reduced. Thus, it appears that seasonal gas substitution
may be cost-competitive with other control methods, in this case an optimized
combination of switching to low-sulfur coal and FGD,in some states.
28
VII. GAS SUPPLY FOR SUBSTITUTION
The seasonal gas substitution model has not considered gas deliverability
constraints which may limit the amount of substitution that occurs within a state as
specified by the model. A gas deliverability constraint would occur whenever the gas
supply infrastructure lacks the necessary capacity to meet the incremental demand
imposed by a level of gas substitution, or if total gas production is exceeded by the
incremental demand. In order to utilize gas substitution, the utility must access its gas
supply from a gas distribution company's or gas transmission company's
high-pressure
pipeline.
Tran
ission capacity can be expanded, but this may
increase fuel costs, which may make gas substitution les competitive relative to other
control strategies.
Becausethe primary use of natural gas is for space heating, summer demand is
lower than winter demand in nearly all states. This condition favors seasonal gas
substitution, but not in an unlimited or universal pattern. The ratio of summer sales
volume to winter sales volume averaged 49% and ranged from 33% to 103% in the 31
eastern states and DCin 19B4(Table 9). The winter/summer sales ratio is only an
indicator of general capacity and cannot be relied upon as a derinitive measure of
excess capacity availableto every generating unit within a state. For the purposes of
this study it is assumedthat the difference betweenwinter and summer consumption
is an approximate measure of available capacity. The aggregate difference between
summer and winter volume is 2030 billion cubic feet, which would provide
approximately enough gas substitution for a 37% reduction in deposition (from Figure
7). However, not every state has the necessary surplus summer gas required at all
levels of deposition reduction. For example, for a 25% deposition reduction, only 14
states have the surplus needed to supply their share of the model's solution. The
summer surplus is estimated from Table 9. column 4; the incremental demand for gas
in each state sufficient for a 25% reduction in deposition is shown in Table 10.
Whilethe availability of natural gas is a significant factor, as supply constraints
are not included in the model. This simplification is made because the availability of
gas is difficult to estimate within a state or at a given plant. The determination of
availability is left to those considering gas substitution. A general approximation of
gas availability can be made by comparing seasonal sales volumes for selected gas
transmission and distribution companies. Table lla shows the ratio of summer to
winter sales volumes for major interstate transmission companies operating in the
29
eastern US.; Table lb shovs the ratios for several gas distribution companies. It can
be seen that excess summer capacity is generally available, but the amount varies
greatly between companies and regions.
30
VIII.
OTHER COSTS
Preliminary findings concerning incremental capital and operating costs for
seasonal gas substitution reveal two significant points. First, capital costs are low and
implementation is quick. Using as an example the Boston Edison Mystic *7 unit, an
oil-fired generating unit that converted to seasonal gas use, the boiler modification
and gas supply construction cost $3.5 million for the 565 MWunit and was completed in
approximately one year (Boston Edison, 1965). This is approximately $6/kV. In
contrast, capital costs for limestone flue gas desulftrization (scrubbing) are between
$175 and $317/kW (Miller, 1983), and have much longer lead times. Second, ash
generation is reduced. If the variable componentof ash disposalis significant, there
is a potential cost saving from seasonal gas substitution. For example, a 500 MV
coal-fired unit might produce 130,000tons of bottomand flyash annually. If variable
disposal costs are $10/ton, the ash disposal cost is $1.3 million annually. Seasonal gas
substitution could save one-half of this sum. The present value of these savings are
close to or may exceed the capital costs associated Vith seasonal gas substitution.
The crucial determinant of the cost-competitivenes of gas substitutionis the price
differential between gas and coal and gas and oil.
Since long term prices are
impossible to predict with certainty. gas substitution is regarded as being financially
risky when compared with other control methods. Actually, gas substitution my be
les risky than more capital intensive control methods. Because there is relatively
small capital investment associated with gas substitution, a utility could easily abandon
it if a more cost-effective solution became available without forfeiting a large
investment. Because of the large capital outlay needed for scrubbing equipment, a
utility saddled with an expensive scrubber is financially limited if it wants to exploit
less expensive control methods that may become available.
31
IX. CONCLUSIONS
Nearly all of the "acid rain" policy and policy analyses have focused on emission
reductions and the cost of controlling emissions. But it is deposition, not emissions per
se, that matters. Monitoringhas shown that depositionrates are significantly higher
during April through September than during October through March for equal
emission rates. Based on this evidence, it is more efficient to control emissions (and
hence deposition)during the summerhalf of the year.
Seasonal substitution of gas for coal or oil is a reasonable option for utilities to
control S02 emissions and is vwellsuited to comply with policies which focus on
controlling deposition. However, it is not a panacea for reducing S02 emissions. The
quantities of gas needed for substitution in order to make total emission reductions of
more that a few million tons per year in S02 emissions would exceed existing capacity
in many states. Some generating units are located too far from a gas supply or face
fuel cost differentials that are too large to make gas substitution economically
competitive with other control methods. On the other hand, many generating units do
have access to sufficient quantities of gas at costs that make gas substitution
competitive with other emission control methods.
The cost-effectiveness of any control method should be related to its effect on
deposition rather than its effect on emissions. One ton of S02 removed in the summer
half of the year has a greater effect on depositionthan reducing that ton year-round.
It was shown that in terms of equal depositionreductions in the Adirondacks.that the
costs of seasonal gas substitution and some year-round controls may be comparable in
some states.
Conclusions:
* In some states seasonal gas substitution may be economically competitive with
other control methods for achieving equal annual deposition reductions in sensitive
areas.
* In some states gas deliverability and supply may limit the amount of gas
substituted.
* Capital costs for gas substitution are very low relative to FGDsystems.
32
References
Bardin, D. J., 1985. "Real and Imagined Restrictions on Electric Utility Fuel Use of
Natural Gas."Public Utilities Fortnightlv March 21, 1985.
Boston Edison. 1985. Personal communication vith Mr. Paul Kingston. Operations
Supervisor, Mystic *7 unit, July 1985.
Boversox, V. C. and G. J. Stensland, 1965. "Seasonal Variations in the Chemistry of
Precipitation in the United States." Paper 85-6A2 presented at the 78th Annual
Meeting of the Air Pollution Control Association.,Detroit, MI.
Elkins, C. L., 1985.
Control Association.
"Acid Rain Policy Development." Journal of the Air Pollution
No.3.
Energy Information Administration (EIA). 194.
Utility Plants 1983.
Costand Oualitv of Fuels for Electric
Energy Information Administration (EIA), 1984b.
Natural Gas Monthly. January
-December 1964.
Energy Infromation Administration (EIA). 195.
Electric Pover Monthly January
1985.
Fay, J. A.. D. Golomb, and J. Gruhl. 1983.
"Controlling Acid Rain," MIT-EL 83-004,
Energy Laboratory. Massachusetts Institute of Technology. Cambridge. MA 02139.
Fay. J. A.. D. Golomb. and S. Kumar. 1985.
"Source Apportionment
of Wet Sulfate
Deposition in Eastern North America." Atmospheric Environment. 19. pp. 1773.
Fay, J. A. and J. J. Rosenzveig, 1980. Analytical Diffusion Model for Long Distance
Transport of Air Pollutants." Atmosheric Environment
pp. 355-365.
Golomb. D.. J. A. Fay. and S. Kumar, 1985.
"Seasonal Control of Sulfate Deposition."
Paper 85-lB.8 presented at the 78th Annual Meeting of the Air Pollution Control
Association. Detroit. MI.
Kumar S.. 1985. "Modeling Acid Deposition in Eastern North America." Sc.D. Thesis.
Massschusetts Institute of Technology, Cmabridge, MA 02139.
Miller, M. J., 1985.
"Retrofit S02 and NOx Control Technologies for Coal-Fired Pover
Plants," Paper 5-1A2 presented at the 78th Annual Meeting of the Air Pollution
Control Association, Detroit, MI.
Morrison, M. B. and E. S. Rubin, 1985.
"Linear Programming
Model for Acid Rain
Policy Analysis." ournal of the Air Polution Control Association. 35. No. 11.
33
National Acid Precipitation Program (NAPAP),1983. "Historic Emissions of Sulfur and
Nitrogen Oxides in the U.S.from 1900 to 1980," EPA-600/7-85-009a.
Office of Technology Assessment (OTA), 1985. U.S. Natural Gas Availability: Gas Suooly
Through the Year 2000. Washington, D.C., U.S. Congress, Office of Technology
Assessment, OTA-E-245,February 1985.
"Concentrations of Some Ionic Species in Long
Island, NY Precipitation in Relation to Meteorological Variables," Water. Air. and Soil
Raynor, G. S. and J. V. Hayes, 1982.
Pollution. 17, pp. 309-335.
Wilkinson, P., 1984. "Seasonal Fuel Switching to Natural Gas:A Logical Approach to
Reduce Sulfur Dioxide." Gas Enervy Review. 12, pp. 11-17.
34
Table 1. Transfer Coefficients for Adirondack Receptora
(kilagrams sulfur per hetare deositad per terun
State
(Tij)WI
(kg S
DC
0.2579
0.1742
0.7903
0.6007
DE
0.6274
FL
OH
0.2300
0.2988
0.2805
0.3117
0.1852
0.3356
0.1650
0.7230
0.6287
0.5225
0.4045
0. 1373
0.2068
0.2033
0.4397
0.8752
0.7373
0.9127
0.4641
PA
-0.6339
AL
AR
CT
GA
IL
IN
IA
KY
LA
MA
MD
ME
MI
MN
MS
MO
NC
NH
NJ
NY
RI
SC
TN
VA
VT
WV
WI
0.7230
0.3486
0.3027
0.5310
1.2950
0.4979
0.2500
(Tij)su
ha- 1
sulfur emitted)
(Tij)AN
/TgS emitted)
0.5908
0.3627
1.8720
1.6180
1.6360
0.4323
0.6938
0.7104
0.407
0.264
1.198
0.995
1.198
0.330
0.473
0.470
0.8085
0.4153
0.8671
0.3120
0.524
1.6480
1.086
1.033
1.6780
1.0410
1.0980
0.297
0.560
0.237
0.758
0.688
0.2772
0.4390
0.214
0.4641
1.1200
0.325
1.7890
1.8810
2.1840
1.2940
1.7750
1.6480
0.8223
0.7463
1.4130
2.6180
1.3710
0.6106
1.217
1.167
1.366
(a) Kumar (1985)
35
0.315
0.723
0.792
1.061
1.086
0.556
0.494
0.882
1.654
0.842
0.415
Table 2. Annual Sulfur Emissions from Coal- and Oil-Fired
Power Plants in the 31 Eastern States and DC 1983
State
Million Metric Tons Sulfur
Coal
Oil
Total
AL
0.241
0
0.241
AR
0
IN
0.032
0
0
0.027
0.206
0.379
0.088
0.544
0.690
0.032
0.024
0.001
0.034
0.302
0.379
0.088
0.548
0.690
KY
0.551
LA
MA
MIl
0.014
0.035
0.088
0
0.273
MN
0.071
MO
0.548
MS
0.041
NC
PA
0.165
0.023
0.039
0.104
0.932
0.712
RI
0
SC
WV
0.082
0.303
0.056
0.205
0.453
0.024
0.001
0.007
0.096
0
0
0.004
0
0
0
0.067
0.007
0.005
0
0
0.001
0
0
0.008
0.007
0.103
0
0.015
0.001
0
0
0.003
0
0
0.165
0.031
0.047
0.207
0.932
0.727
0.001
0.082
0.303
0.059
0.205
0.453
TOTAL
6.906
0.350
7.25
CT
DC
DE
FL
GA
IA
IL
MD
ME.
NH
NJ
NY
OH
TN
VA
WI
Million Tons S02
Coal
Oil
0.536
0.070
0
0
0.060
0.458
0.843
0.536
0.070
0.054
0.001
0.076
0.197
0
0
0.054
0.001
0.016
0.213
0
0
1.210
0.010
1.534
0
0
0
0.149
0.015
0.011
0.001
1.220
1.534
1.225
0.551
1.225
0.014
0.101
0.095
0.005
0.273
0.032
0.077
0.197
0
0.606
0.157
0.071
0.549
1.218
0.041
0.091
0.367
0.512
0.088
0.230
1.007
15.346
0.777
0
0.182
0.674
0.123
0.456
36
0
0.002
0
0
0.017
0.016
0.229
0
0.033
0.002
0
0
0.006
0
0
2.071
1.583
Source: EIA, 1984
Total
0.671
0.843
0.197
0.032
0.226
0.212
0.011
0.607
0.157
1.220
0.091
0.367
0.068
0.104
0.460
2.071
1.616
0.002
0.182
0.674
0.130
0.456
1.007
16.123
Table 3.
Summer and Annual Sulfur Deposition Contributed
by Power Plants in the 31 Eastern States and DC
kilogramssulfur per hectare per year (kg S he yr 1)
State
Summer DeDosition
Coal
Oil
AL
0.071
0
AR
0.006
0
CT
0.023
DC
0
0
DE
0.022
0.006
FL
0.044
0.021
GA
0.132
0.018
0
0
0.193
0.001
0
0
0
MD
0.279
0.239
0.002
0.029
0.074
ME
0
MI
0.150
MN
0.010
MO
0.127
MS
0.009
NC
0.092
NH
0.021
NJ
PA
0.037
0.113
0.603
0.632
RI
0
SC
TN
VA
WI
0.034
0.113
0.039
0.063
WV
0.311
0
0
TOTALS
3.464
0.258
IA
IL
IN
KY
LA
MA
NY
OH
Annual IDeposition
Coal
Oil
0.001
0.055
0.006
0.003
0.098
0.008
0
0
0
0
0.029
0.028
0.068
0.179
0.026
0.256
0.362
0.309
0.003
0.038
0.007
0.032
0.091
0
0
0
0
0
0
0.188
0.015
0.178
0.012
0.119
0.028
0.046
0.007
0.007
0.113
0
0.013
0.001
0.002
Source: Calculated from Tables 1 and 2
37
0
0
0.002
0
0
0
0.073
0.007
0.004
0
0
0
0
0
0.009
0.008
0.141
0.141
0.738
0.756
0
0
0
0
0.001
0.016
0.001
0.045
0.150
0.049
0.085
0
0
0.381
0
4.401
0.333
0.003
0
Table 4. Average Cost of Natural Gas at Electric Utilities
in the 31 Eastern States and DC 1983
State
$/106 btu
AL
3.129
AR
3.21 1
CT
5.930(b)
DE
4.180
DC
4.480(a)
FL
2.529
GA
IL
IN
4.177
5.291
4.238
IA
3.747
KY
4.551
LA
3. 150
MA
3.887
MD
4.480(a)
ME
7.660(b)
MI
4.38
MN
3.798
MS
3.325
MO
4.164
NC
4.860(a)
NH
6.000
NJ
4.046
NY
OH
PA
3.932
5.169
5.104
RI
3.753
SC
TN
VT
VA
WI
4.285
3.870(a)
4.220(a)
4.202
4.284
WV
4.546
(a) Average prices calculated from data reported on Form EIA- 176.
(b) Average 1983 price paid by industrial consumers.
Source: EIA, 1984 and EIA, 1984a
38
Table 5. Summary of Results for Seasonal Gas Substitution
% Reductiona Reduction in
in Deposition S02 Emissions
Marginal Cost Average Cost
of Deposition of Deposition of Emissions
Total Cost
Average Cost
of Deposition
tion
Re
Reduction
Reduction
( 109
( I0 $1 983) ( 0' S per
(1 06 tons)
per
kg S04 ha 1) kg S04 ha I )
0.6
Model
-.204
-.165
.456
Reduction
($S per ton
502 removed)
-340
102
1.3
.610
.233
.708
469
15%
2.2
1.671
.418
.853
759
20%
2.9
2.929
.542
.938
1010
25
3.9
4.403
.641
1.093
1129
30%
4.8
5.858
.721
1.231
1220
35%
5.9
7.867
.827
1.600
1333
40X
6.9
10.931
1.002
2.996
1584
% Reduction
in Deposition
O3mSubstituted
(billion
cubic f)
Coal Displaced
( 106 tam)
Oilislaed
( 0 brrels)
S per 106 btu
dsplal
337
8
79
-. 303
10%
501
20
84
.609
15%
709
37
87
1.179
20%
909
53
87
1.611
76
87
1.873
5%
25%
t1176
30%
1440
97
94
2.035
35%
1795
127
95
2.192
40%
2396
176
97
2.281
1
(a) Calculatedfrom 27.5 kg 504 ha- baseat Adirondack receptor (Fay et al., 1985).
39
Table 6. Gain Factors for Seasonal and Annual Gas Substitution
% Reduction
in Deposition
(summer
3 Ruhicton
3 Rductonb
in Emissio
in Deposition
nlin Factor
Effettlveness
(yer- round
controls)
(a)
(b)
(c)
(d)
Summerc
Oin Factlor
controls)
5
3.1
3.3
1.61
1.06
52S
10
6.7
6.5
1.49
0.97
543
15
10.5
9.6
1.43
0.91
57%
20
13.6
12.7
1.47
0.93
583
25
17.8
15.7
1.40
0.88
59X
30
22.1
18.8
1.36
0.85
603
35
27.2
22.2
1.29
0.82
573
40
31.9
25.4
1.25
0.80
56
Calculated from bee of 22 million tons of 02 emitted annWlly.
Calculed from Eq.(2).
Equals % Reductin in Depositin(summer controls) divided by % Reduction In Emissions.
Equals % Reduction In Depostn(ye-round
controls) divided by % ReductonIn
Emissims.
(e) Summer coontrolsare XX more effctive th
at an Adrondack rceoopr.
year-rounmdcontrols for reducing depoition
40
Table 7. Comparison of Costs of Deposition Reductions
at an Adirondack Receptor
S04 Deposition Reduction
%a
kg S04ha-ly-I
Avg. Cost of
Deposition Reduction
(1983 109 S kg
I
ha- 1 )
Morrison and Rubinb26
7.2
0.25 0 -.4 30 f
25
6.9
0.641
Seasonal Gas
Substitutionc -
-----------------------------------------------------------------Morrison and Rubind30
8.2
0.46 0 -.68 0 f
30
8.2
0.721
Seasonal Gas
Substitutlone-
(a) Based on modeled (uncontrolled) deposition of 27.5 kg 504 ha- I y- I
(b) Stafford Bill, state-wide emission cap of 1.5 lb./ 106 btu.
(c) Summer gas substitution
model set to 25% deposition reduction.
(d) Mitchell Bill, state-wide emission cap of 1.2lb./ 106 btu.
(e) Summergas substitution modelset to 30%deposition reduction.
(f) Calculated from Morrison and Rubin (1985).
41
Table 8. State-Level Average Costs for Achieving Reductions in
504 Deposition via Seasonal Gas Substitution in Electric
Power Plants in the 31 Eastern States and DC
State
25% DePosition Reduction
30% Deposition Reduction
Avg. Cost
Avg. Cost
Dep. Red.
Dep. Red.
(109 $ per
(09
per
kg S04 h( 1)
kg 504 he- I)
AL
CT
.828
(a)
.851
1.187
DC
-.882
DE
.777
FL
-1.072
GA
IA
1.115
.968
IL
.854
1.024
IN
1.009
MA
.911
.837
.128
MD
.814
.814
MI
.995
.949
.859
.473
.066
.724
.796
.995
.961
KY
MO
NH
-. 882
.777
-1.072
1.130
.968
.840
.128
.859
RI
-.403
TN
.830
VA
-.235
.473
.066
.768
.842
-. 403
.873
-. 235
WI
1.023
1.106
WV
.707
.806
NJ
NY
OH
PA
(a) CT is not included at the 25% deposition reduction level.
42
Table 9. Natural Gas Deliveries to Residential, Commercial, and
Electric Utility Consumers in 31 Eastern States and DC
State
Summer
Volumea
(bcf)
AL
AR
CT
DE
DC
FL
GA
[L
IN
IA
KY
LA
ME
MD
MA
MI
MN
MS
MO
NH
NJ
NY
Winter
Winter
volumeb less Summer
(bcf)
(bcf)
Summer Volume
as a Percent of
Winter Volume
21.9
37.9
20.2
55.4
68.7
39.8
33.5
30.8
40
19.6
51
8.1
9.5
1.4
85
9.7
112.4
20.9
11.2
46
109.1
-3.3
103
55
43.8
107.9
64. 1
41
202.5
510.5
308.0
40
65.6
173.0
107.4
35.6
96.6
78.4
234.7
61.0
49.9
38
37
36
28.5
233.7
0.5
1.1
71.9
11.0
0.6
95
47
33.6
73.8
115.1
152.3
370.0
46.6
49.8
51.3
2.7
134.3
38.3
41.3
217.7
87.7
59.6
9.8
137.0
85.7
3.4
79.3
45
64
41
35
OH
PA
RI
111.4
208.6
410.5
47.2
377.6
282.2
10.1
16.1
170.8
6.0
84
37
44
62
62
39
37
39
62
SC
TN
VT
10.6
21.1
0.9
25.7
63.5
15.1
41
42.4
33
2.2
1.3
VA
WV
24.1
27.4
42
47
16.1
51.5
43.2
Wl
48. 1
119.1
71.8
37
40
2050
49
NC
Total
129.3
256.1
18.2
141.1
2007.6
6.1
4057.6
154.4
29.0
236.5
27.1
Source: EtA, 1984b
(a) Summer--April through September
(b) Winter--October through March
Note: Industrial gas consumption is not included here as the data is not yet reported by the
Energy Information Administration. Exclusion of this componentprobably causesthe ratio of
summer to winter volume to be slightly overstated here.
43
Table 10. Natural Gas Substituted for a 25% Reduction in Deposition
State
Gas (Bcf)
Sufficient
Surplus(a)
AL
51
N
DC
1
Y
DE
!9
N
FL
144
N
IA
IL
3
20
Y
IN
73
Y
KY
61
N
MA
59
40
50
25
9
N
Y
MD
MI
MO
MS
Y
N
Y
Y
NH
11
N
NJ
30
Y
NY
Y
OH
137
143
PA
184
N
1
Y
RI
Y
TN
41
VA
3
Y
WI
2
Y
68
N
WV
Y
1176
(a) The difference between winter and summer volume of sales in used as
an approximate measure of summer capacity. If Table 9, column 4 is
greater than the incremental demand shown above, then Y.
44
Table 1 1. Summer and Winter Gas Sales Volume(a)
a. Interstate
Pipeline Companies
Summer Volume as a
Percent of Winter Volume
Algonquin
Columbia Gas
Consolidated Gas
East Tennessee
86
50
65
65
El Paso
95
Florida Gas
90
Great Lakes Gas
177
Michigan Consolidated
Midwestern Gas
Natural Fuel
Natural Gas Pipeline
Northern Natural
Panhandle Eastern
Southern Natural
46
83
47
70
50
57
65
Tenneco Inc.
86
Texas Gas Transmission
Transcontinental
Trunkline Gas
United Gas
73
72
58
82
b. Selected Distribution Companies
Northern Illinois Gas (IL)
Peoples Gas (IL)
K Indiana Public *efvice (IN)
Indl*ia Gas Comny (IN)
Louisville Gas and Electric (KY)
Columbia Gas of KY
41
38
60
47
39
34
Boston Gas Company (MA)
85
Michigan Consolidated (MI)
Consumers Power Company(MI)
PSE&G(NJ)
46
44
43
Brooklyn Union Gas (NY)
65
Consolidated Edison (NY)
54
(a) From EIA ( 1984b) and State Utility
Commissions
45
0
U.
Z
oToCo
4-
*4-)
co
oaZ)
I-
.o
Co
'
c/)
.n X
0
X
O
I'
'
0
I'
O
(1L4/68)NOIlWSOd3C 13M VOS
46
0
-
S_\\\\\\\\\\\\,,\?
z
fi\,\\\\\\\\\\\\:
\\\\\\\\\\\\\\\'`\\
\\\\\\\\\\\\\\\\\\
t,\\\\\\\\\\\\\\\\\\\\\
fi\\\\\\\\\\\\\\\\\\\\
-0
- V)
L,
0
oo.
00
C
O
co
4~
0-
r
II
L,
.(
0)
\\\~,\\`
I
0
to
0
V
m
a)-
0) LL
\,?\\\\\",\\
c-
r-o
·
*
s
0,
0
C-)I
4-
U4D
to
O
L,
U-
a)
LL
c
I.
()
-
O
r-
0I
?\\\\\\\\\\\\\\,\i
I
I
I
I
I
I
I
I
O 000
0)
0
0(D
0Iun
0
0
0N
0
I
-
Slnoq;PMO
L L)
UO LL ,1
47
0
I-
L1
U)
vE
2
Z
-
o
,
4U)
O
a
3
0
)
4-:
U...
oZ
o
C)
U)
o
O
0
0
0
0
0
0LO
00
LO
-
1-
ri
1)
N
N
O
u
0
0u
($86L)
nl9 uoLlLW ad
48
0LO
0
41
. .
*2n
*_
=
210
200
190
180
170
160
150
"
140
M
130
=
120
-
110
-
100
m
Sa,
90
80
70
60
50
40
30
20
10
0
J FMAMJ JASOND
J FMAMJ JASONO
1983
1985
1984
Figure 4a.
V-
J FMAMJ JASOND
Natural Gas / Coal Price Differentials
A
11.
t[
-10 -
tlt~EiE~Et-lt~f~f~~E1BM~
IH
3r 3F
I
-20 -30
mr
-
-40 -50 m
-60 -
0
._
-70 -
I'
I
U
i0
-80 -g0 0.W
tI
[
-100 I I
-120
I I
%13V
1
-140
150
I I
1
I
I
!
I
1
1 1
I J
I I I
I
I
I
I
I
I
I
I
I
I
I
I
J FMAMJ JASOND
JFMAMJ JASOND
J FMAMJ JASOND
1983
1984
1985
Figure4b.
Natural Gas / Oil Price Differentials
49
t
Ct
Ln
)
o
4-)
4
u
a
U
aa
4-
O,
4-
Q)-
o
tn=
40
0L-o=
0
0
Lo
rr
LO
0
0-))(
O
.
n
r-
N a
4-
o-
)
00
£86L$
4
SUoLLLLS
50
nro
-
o0
r
4'
0
id-
.i
0
4r0)
C)
C
0
4
S..f
o on
0)
)
a)
cN~ L4)U
._
0f
c
> .-
*'
Q)
-o
ww
S..OV
0*)a
0~~
,- ,- 00
I- (D
C ) d- I
N -
0 d
L-
eq OS 61
ad
uoLLLL
51
N
cv
r3
Q '
O
Ln
v
a)
a,
0C
0
O
*_ C
r
o
0.4rif)
D
·( r
L
o
=
IC)
N
o ,
cN N c
L-
U4 OS 61 Jd
-, O$ UOLLLL
52
6c
o0n
L)
O *1-)
4
(n
0
r
o
I
0.
· r
4-
O
o
Ln
0
Co
4)
r-
LO 0 " ra)
0
C
)
CC
1c
0
O
00
LI
0
L)
.CD
LI~)
t.
1
(D.
LO
.
a
f)
0
O4).*
6
(sw3 %/IdaG%) Jo;e2j uLPeg
53
0
6
-
Ln
0
4-)
'4)
LO
o
oaQ
CK
O4
O
C 0
0
4
iDC
0
uO
Ln
N~N N
a.:l-3'.qn.u LLL
;aaj
Lqnouo LLAli
54
O
a
V
/-
-
o
.0
o
U
)
CDC
o
o
:rCL
._
U)
n
o
rO
In
r)
o
0)
O
*.n
)
0
o
:
C;
00")
LO
0 0Q 0U 00'4- 0to 0N 0 00 0 ( 0O 0 ", 0Q 0 0'- 0 0N 0- 0
0 ,
[eO
0 SUOJ UOLLIW
55
rl
-0
r0
!0oo
4
*
U
4
4-
4.)
o
A,
)
0D
C0
(v
o
'
0
0
a
.
LOE~
a)
.~
°*
- ©) u) 't n 0, -- 00
OOC
M 0 uL
0
L.O
0
S L~4aeS
.LW
uo L L
56
0s
-A 0
0
I'
v
r)
Q)
.) C
o
L.
C
u,
C
U
U,
cI
OCLc
0 c0
W
4-
O
*
rU
(O
r
L)
ZOS J0 suol
,0
UO LLLW
57
CY:
0r-)
I
In
c0
0~
0 '
04)
0)
0
O
W0
n
0
r-
I)
0J
*n
0
o0
·0)
LO
0o
Ln
-' .
0
0.0
4-
.- L.
Ua t
0-
0
-
I
O
I
n
I
I
Nr-
I
I
I
I
o, M
I
I
I
I
I
I
( n ' rI-
paDnpaeZOS 0 uol jad $ puesnoJl
58
I
- 0
I
I
+ l
I"'
i-
'I
c
N
0
c-)
0N
.- )
00
o
*r0
o '
c.,
CO
ci
0
o
()
c0
4*-Jr" r-
0c
00
r(
O0
--
I
(b
a)
._
U_
0
N
r_
-
.;
(0 d
.
N
.
£R
L-
e4q
O
OS 6
N
t
1
I
66
ad
$ uoLLL9
L-
59
(0
I
I
N
00
0N
._
-o
t
*
uO)
0
0·
S-
C
0
-
E0
EO
r
Q,0O
_
OL
0
080
L)
0)
Ia)
Sr-
=t
v
O
6666
O
I
(0
L-
L-
N
L-
O
N
i
I
I
6 6
(O
I
q t OS 6M Jad $ UO~LLLS
60
o
I
N
APPENDIX
A
Datafor Coal-and Oil-FiredElectric Utilities
in the 31 Eastern States and DC
GuideColumn:
(1)
Company name
(2)
Plant name
(3)
State where plant is located
(4)
Annual coal consumption in thousands of tons
(5)
Coal price Si 106 btu
(6)
Coal price S/ ton
(7)
Sulfur content of coal, percent by weight
(8)
Coal heat content, btu per lb.
(9)
Annual oil consumption, thousands of barrels
(10)
Oil priceS/
( 11)
Oilprice $/ barrel
(12)
Sulfur content of oil, percent by weight
106 btu
61
NNN X~itw~ainn
.M~~
CDOJIX0
MN_
N
.
..
-9L
i0L --
n i-i
0DfN
LO DY~
N ^
iZILOtM
t~~tlfl~
U_
m~N
NO N
:
..
,gg
N
.....
M
I
N
.
N
~
M1
.
.
,NNWcurN
Ltsa
.
.
r@;c1
~
(r
z
.
m
SN
.
~
mt
-r
..
c-L
r~c @CD
cu
ir IrC^^r1;
N
i
N.N
.
0QU~~~~~~~~~~~
t .eNit'
to
nmc
N
ost
gineM + -
IN-LnO
_L
OF
u VDs
N
~ ~W
^0
N
t111
6
.
..
C~~~~~~~~~~~~~sC~~~~~~sCCSQUUSU~~~~~~~~~~~~~~~~~oD=D~~~~~~~~~lLIL
Ian
2
MI
u I- LLLC ILiLLILILIL~~~~~~~~~~~~~~~UH ffliYff
ims ILIL
In ^N
-tIN UA .
U W
CCCCCCCIJJttFFJUUZUU>JJJJOJJ
,-
a
Hnu~ftL
CLILWIla)
QE##-9
af
x uu.. 0j I-.HU
IfssK+N-v
S s>HD
cluzW
i i-zo wxFzJ
3 I^v
s~~~
-w
t~~~~W
-C
J
U
YE
UO000
SS
^
Y~~~~~~~~~
0 w I..4
BX
53U3wt
W&
JU
Zm~
wucp
ofo
Z
i~SQLilWJ3F
~uI
E
;
era2s
ca
62
-
O£C(W-£Ff
z
Z~c UuuWlul
Q JJJJW
%i
S;
N
mwVOra z
u
n
S|Fa~
gr
U
o
w
8
cJ
t
rI
u
a;
D#2t
C
d
0FFirOu ilJJ|JJJJXJJ
uusswoooo9
OLWI
%Dnl
CD
mD
-
CNN-
%0CD
t
IL)O'
0%
0
0
(N
.j 0
.4.
-
SR
_'
N
-NX
h
hN
pt
2
-
NN K
4NtN
d
N
D
NN
VE
_
NN
. . .
tttt
tt
.
t. tt
hf
JJ WE
0 -0
U
-a
MUM
NI
N
"
XD~tZ
ttrD
- V V
%aD
D_
_N_
_.-
.gI-.
U
__,
N
LL
l
N
%a
N
0N
MtlOU)-lOeNNNUNNONNODN
. .
. .
. N nn .
..
. . . 9. . . . . .
in)
t
t
...
. . ..
t V
* 0n
.9 . .
NN
-N(NCD
U)S
_X^DX
9.
9
N>
- NN N
NN-tM
g
T
t
0 o
fijlN
NK)N- IasUOU~rpD<DS~w=-O
-tN'D
UtNN(Vl
hfne
OsInN.
NtAlN
w t N VI-N(NDON
K)NtN--4
z
U7t
*
INOMWON9-c
D
N_
N
sNNnA
_______
______
4
NNIMZ -4N--- SI-N-N(IInNO
Nd INN
-
O.
In
>Hi
.
..
C O IS)-NN
Ir(L
_4
J~"U
In
W
m1
i
ll·
.. .
.
-4--4-
.
i
.
N
.
D
i
. . I
-4-4N-----
i
.
. .
T Ni
.
.
.
dJi~~~~JJXiiii~iiiiiiiiCcirwct
of
.
z
xiX3P-aZ* NdNttSrr
eIi~
,,0E~~
IY
I-
LS F
tN
Z
I-. 0
Of
F Fd
QWW"uduuu
.1 WWO
of a
2'I
8
- I| kL IC
&I
d vl oi v\ffii
a
0 UU
wwj Jj
_
3 _zsu
-i atAl
04
Y IsJzascrsxts
ttx(c
;Q10 :I r0.0.
z
wF*~st
W
U
LL
ofLOOOZ
btt
BBB
.,,@XF
L)*1tW~a!
b5-~~~~~~~~~
5 i~s##IF~i!
spbgg
g 1 El°J
~~~Eacaa
ag~~~~~~~~~~~~~~~a
b" w w "U
63
0%
%D
N4 LL
, 0
-O
- I"
in
0F
0
O\
b.4
'"
NInltWt IN tt@Wnt)NINN
'
I wo OO~~wt~^
Am __v
,
N
C)
in
t
N.
14
x9V V I C---
I'Ig~~~~
Q _-i'I4
u
tNN-N
WN".4Ž
-----1D I -D~-
"
m
N 91~~n~N99~~!~.~ N-!iWf4N
to in
W 11'W
1'
(DO
CD-NO
U*
F-X X
t~
D
@-
01wt
-----
D
NN
I
___
-
N OW
VID R It I x^N
§
- < I _It
-4
(ON
tH
--
NN
m
t N-C
t)tDO
D
N+ N N N N--
N
It
iID1MN-uNii
15 DNNNXrsNI
t@XO@N-OfOCC@@
m - m 19
N.Ng
D@t__I
0
0t~
O
WNNNNNO
0+i
r,N^-N
N * ws
DE Dz
r)
N ^^^-^N
6>61XD^DO>^DO St<
.
.~
.
c
..
..
*.
a (OY+tXOD
-; N
_
Z
DW
ZCCCCSJJJJJJJJJJXJJJJJJJJJJJJZZ
Y)D~~~
I W$
IL
p ,
u C~~~~~~t~C
wJ
WZ
N
IL
DIL
Zd
Ln
I
MM-
Y"W
Y J
00
d
Iof
J
CI4
M
22
0H-H-
2
"-UUUUUUUU
64
i
1LIIE
mmu00000000
0.0
I -W
I d-..J
..J.J
w
Ir~
Z0000
-_JZ
4..JJ Z
L~~ooW
Z Z ZZ Z
.Z. U
WLUWWWWWWr
ii
HH
I
-Z
NI
Iit'fisY
l~,nms
OOODOO
0.0
9
)-CC3
m
I-L~lP
~
owt-
ZM
-J
J
Yi~i8£XW
LL
Y
CI(
-JJ-J
X
Iw
UUUJUW
UM
M
-e-'
fiO
UL
J:
No3
Ji
rui
X*
Ogg
040
.;~
in~zxJ ND----Vww-O NN
c0
OI@@0N
m
~
_
N
DM
P4 0404
N-'4 No'
O<0
-
N- in *
eN~@T
O
~ -Dt-- ~HONtOtWN
To
"
4Iflt
N
MXeO
NN
1<zNnftIN0N^0NNNnh)-W'4t4'40N'4'.V)
u:...............................
N~~-j
6HW
ll~ 0.>i
wl Q
flP -NJR
~StcntDD
t@nn6@9@@GLFL@80220KhE-h~
h@g
O
c
S
·
,
104N
gm
Nn
t
(Drtcrrr In~(~(r~~~r+
Q~C~W~
F
-
2
n
iii(PnSI
a"- "Z' I-ua-
on' Eu"
nm
NwzZZZZZZZZZZZZZZZZZm"mm"Uzw
0
N40
N4 Nuvuvv
ntuN
0
ape^^^^^^^^^^^YYYYYYY
v
YYYYY
0iEN t
it
~~~~U } J
%L
WJ-I~fFLQ~c+ccyr
ljZ~~a AiiS
IwZI ~' QltL
%
-h
IC
-da~a,2
sro
NN
Ur~dun(
= -NSYr
SMi
X
npre
-
@@ttL
v
1. -
32l
+u,
86
CL:::
-( C
Q
. i~flI
~
_*Y IrZZZZrZ8°8
uen 11 zzzttYYYYYY
oogD9@wSwuruuyu
u
-Jii 9991"S
zz~zzxza.
im~
jM *
^wv^zzLXLo00@XSSYyS
w~~~~u0044004WHJ
~~04dd~~L
Nd
I
(Y
tf
0 AA4
NJLJ(JLJLJ
040404
65
L
o
o
0404b4
."
U)
(D
-1%
N 0
.4 0
s117X
mass_ Nvuz
OWN -NNN
- as
JI
NmmmON
clfbi
NNiON
-0
-
~~ ~
~
@
.. ID-
N
J-
Dma-@
NNibiNNN
{sU:tD
g
\
c
. NiMN
. Xs-.. IN0
4
Na<Xs Wse~~~e~QL
N~~nO
tttO
.
.
.
_t0@~l
vsDu
1@lIRt
N X
-t -t9I
.~2
Q
N
'a
..
..
-NN
.
w
)cC Ir
NN-W
_ N -CD
N 1
ID
L) N CD)I
NNt
nmon
sotwos
NN OIO
N;N.Nt6D6Ii4DN
..
. N6
----
0a
.
.
.
.
.
N - itt
°b
0"ga
666
MN"TOOM
N
In .* I'
_@__VI
6.
.
. **
NO
CD
In
.
-
.-f..40
ai Ge
0d
sS~pmKt
.;
II
g .
.W
..
N
VtVI
" tS. N D
.
3N
in,:-:
r.
_N-NNN
N
~~~~)
t
~ ~Z
c c cc cc c
WUM-O
J
Jd
I
W0
5
g
WCI0
WUr
uE
D
lUw
5
j
UI
W4
YL z.f
Om
z mu
Us
irz~w
Wziu8~ol~rrE
igs ;
Z.JU>§§J8
tnS
J
w
66
N
~~cttwccl
I-
Irrr
u
w~~~~~~~.
W
I
5_j0
tg IL
0ill232m
OP Ccer~wmJi# ; i33
jw~c~ wwwH~
LA
*
N
VI P4
M3155
*
s
N
-N-
N-
*
!s
N
.N
U)~
t
W §M4
6
.
iNsN3N_
S
i UU
0'ON
Di
L
W2 I9OU00
II
i 56Effi
)!t Ix0 01i
CK
'c65 '
N
CD
N~~tL
-o
J
_
NNOcNN
~~~~~~~~.4
_
J
(
CD~~~~a
O~i tn *
%
NNN
CD%D
N
mJ
0
0
N-iO9-V,,t3
V)-M
N
l-tID@
N
t
-D
N
J(L
-j
r
N Nt~~s5@@@@s
N-@@@@@@@--@@@-teV)
C.
.
-~~
- J
cr,
-
-
+__+
wui
~~~~ww
---Jw
C---N bN,N0NN.(-^--N---
...............
w
www@w222wZ
O
--
w__
c
~n~~N
~
9NU1@N->@X@~n-tn-t-
kl
t-
N V) 0
~
@-ZD~M
SY hO9iD
Ug
(DN N
N~~~~0k>J
qD~iN~e
w-5
2 c\
N
J
N
-B~~~0LI
-
tt
te
N~~~~~~~~~
z$o2tE
_n
~~~~w
u~~~~~~~~
of
J 9 I LLn
i wz j J
--
iw
wJ
~tt
rZ ow
FFFFFXZ
VIVIt
I
W LA Y t
s
c
I
HES~~~i0~
J~l
67
"O
-E
d
cc
-
FA
-
OOFFF~~tO
@@@tt00FXQW
t ___t_
tXX
t ~ _zA
~twwi0se
~ ~n
c
Y
&c
lv~-~fifwi
0
Wt8Z
U
um
Lnz
fii
U
- $N " N-
N
J01 00N
2
a'
~ ~*X ~ a
-ii-~
o
WNa'
Z
4i
Ni
u
+A
~
Oiffl
EDL1
OXCR
Nvot
-
O__~l
@ N
Bsu~B~
ct0i~~cc
+_gao^>nN@
y
_
.1
W
Iii ug:iI~iIIz
UUUUC
%At
i ..N'iiii,4ir
a
i_
-1
@>e~~
Y)QI
l
N .. N
.a
01e9-+Nzf0UNZD
o -~~~~~~~C
-__
Nf
nin-Nfi-^W0X@N
a
q1*N11TA&
>JN
0)6f
SN S S.e''..SSSS..S
vl
a'
R n~~~~i
W+~~++U+
inAi'.
NNt
IN,NVt VI
>etW'.N-N0N0
IAN
r@
0
in.....
.4.0M
-NMrKNW'
~ ~~i1PNN
r90lD@>_
in
- t't4SWWW
%D R ONO%
t
CY$
~~~~~~~~~~~~~~~~~~N
N N N
_.
N
*
*
.
*
__m
D
Y
0 n
S
tD
_
u uu;;;fiII
L)
J
at
o .J-A
FSS
0
,-WWW
_. wwzz
Po
N
EiE^lEE
i~u
z
-W.
g1iCaSSUHizzzzzZZZ~iiQ~uZZZ
18!iErtE50ES114
Hoi3l
R -111mg
£:i t9
CYi
zzz
of
IA
68
ww~tZ~tt
i=
N
N
NMMNWa-.tor
N-MMNNNMNW)NNW
W
M)
V
tNDe
N
eJ'4 LL
Ifl
NUDO
tmNOD
D
'
. ....
,,~~~~~~~~~~~~~~~~~~~~~~~~~~~n
JMN
NM 0l
%t
9tNN NN
* 0N
16N-6vJt
t t 0 & IVI%
t tNVI Nrm
t
s-j. NI.o
-N..
. . . . .
0
0
S J
M.
U)N
M
C
-g
.@
NN
N.
Vt D S
f
I
t
C 0n
m
FN
WWON
'4
D
-1
iei~~;i;;;
F (%Cd,-N'.Dr'-eCf Nw
N
m
om
I
:
hN~IL
N
On
-Wr
-'4~
N--4' N'4-
lt
NN
L)~~~~~~~~~~~~~
J~~~~~~~~~~~~~
m
vD
B~~~t
*
^
N
.1
t
NOZ
hilD
wD
lih
0%M~~N9(.......~
N UDtDS
B t S;
^
I- o~~~
_
i BE++W
NN
w
_in
in_
> Z~zouuu uuu u mJjjzzzza*uw
-5S
~ ~ ~IN W ~WQUDWW
W~llZ--gK4
r3
Jw J
ww
seNeo
1FF1e1--~z
Y ww;tWx~wfig
00>u~ultn§§
stx
VI M O
O
(L
9
z
|zl~noooooooooo
w
YwwxLd*3
U
>
tnulenenowww=
IC-
-
§
U10v1I^JOOEOOOOO§O9OO~w^^>>>>>>i~i
gLnLX}lt¢Ottl)JJZZZZZOXE
m
do
www~
· ~
n,',
o ooooo oooo
t
U
g mm
U MWOO
UUUUUEJJJZZZ"
WWE~>>>>>
69
In
uwz HU
..
dd
4 dU
iwUitAiu
UI9JJ9 Out
WWWWW=
80
oJ
0V
In0
t
1
l N ll c
_
X
rr((Neflqt
m
lO- N
t
t
t
I iNllr)QBna)tel-@t>~RAW~WIDCYr}x MEI--I
WD
'4
-Jo 06u
B!
I~Q'y
uPoo
ZNO
wi
-S1--
yu
jX
EMMI (V I. S
"&
N
O%V)
- F
tN, _Ofe
iI
0 i
NJ
Dw
t
J
5^
ooos-1 o
-
X
zs-
F
tiS
& 11
I
"1
i
t0
-, A
y
I
o
I
J
oI
Sd
H~d~ude~osloososos~osEFoonw
70
VI
NU.
Nin
rl
N
CD IOtCD
O
0O~~~~~~~-
"0
.I
H
~K ~ ~
~
~ ~ ~~
J:
W
-0
~
~
* .
N 0
NiXU~W
W
D
'
t9fuigf
ts-tAon
-~r
;2ENNL$N
l
.
NIDN
'E "I0+h
-
I
.
*
0".%
@~
@is~~~~~~~~~'
U
I
ttt
~~~~
tn;$$2T
-Na
-- ^-N^_-NNN
_NN
^_N____
* _N
%a 9K WUW7,
a K
tn
O 111
*
VN
R.;
iS
m
^mer*
0~
n~~~idi
WUNN
wwwd~www
frIX L^ULIUL ..
N
U~~J~~te~D
N
N
N
at
r!E
10W
sFdtIW fiNgFt§ ]
cu22w
ntt!''~33
Ix~~~~I
- -§W;
t$$t$2$$t$t$
ggggggggggg0ggluuuouu
71
UU
J 0dI
W
N
L}LIUXU
94,4g~,4
tt
chnnL
IW~i atz
SE
S
Uu
n~U
"WSWXWbXQZ
WOF:FF
|~ Lf
(40
I 94
U
em,
n
. ..
WWWW
.
ZZ6lCZIr~aYQzs£Cfa
l
~DN
x rN' IDt
c.
il;,QaQQ,
Ncr~~~~~~
)
J>E
_
*VI
tn ~
-ox ~
rcr
0
J
cl
tIYrn
NnaLOi
g(+
rQ
@@-------*-rr
P6~
~
1-11
r
c
os -- o r
1e
N...
·d6~~~~
·"5
l
N
Ira
g
._
..
..
tIN
O'Nol"90
nt-
..
(1N
.
.
Attftp>
.
.
.
.
.
.
~
~
0 t
_n~
9
tb
alll@
8AE
ttg2
NE
x~~~~~
UNN'Iis
u
-1o_
5
%~8.SN5
1~ ~~'1~8
nit'."w.sW
99
'N
It$;$t$t
IeNg
CEA: ml
I
S
ZZZZZZZZZ
2~d
Z3MZZZ2
+n+++~+INN"n~~
·0e
ciicill
+~ JNt_ 64 n _
zzzza
I~~~~~~~~'
*IDMeI
*
las..
5
LN3Nlem
I
II
51
-
'4'4'4'4'4'4'4'4'4'4'4'4'
J 2titd~2!
mm
r*
^^
--
I
--
'
DlN
2X§st"f Id
(Y
:|§:!Ifml!H
I
58i
# 8Pf iiTi--SS>S>Sx
#
3zaaa3
##
#
FFQumo__^rr rF Q>>a:
72
222222222222iii
^___^_
.!
-rO
.'I
0%0
61ff
RAROHRIRIE-N
.
hi
.
.
.
.
.
.
*.
.
9 9 9 9 94 49494_Il
_t
* . . N94 4 N9 94
Bit
0
LJ*
9 0N t
Ing
4
iND
H (1944
44l_^______
tt--mg
H-dl
t 12zFF
Wa
inE
NJ
Ni
isitid I
ws C w
t
gt,|
Ef5
Ii 6
To~o
lulls I 73
APPENDIX B:
Price Differentials for Plants and States
GuideColumn:
(1)
Company names organized by state
(2)
Plant name
(3)
Type of fuel burned. C=coaland O-oil
(4)
S per million btu price differential between the
state-average natural gas price (from Table 4)
and the price of coal or oil burned at each plant
(5)
Billions of btus of coal or oil displaced. or
conversely gas substituted. at each plant
(6)
Weighted (by Total BBtu) average price
differential ($/106 btu) for each state
74
N
-)
N
0)
t
03
U.
(DM. 0
3
-i
.
a.m
0m
W
.LL
-
"
as - D ,
W
v.4
06r
Q\
l) N N N
N
' )0
N -I
W)-
-4
O
U1U
.4 --
U
o
W
0
L U
010
U
J
wmI
W
1
E
W
IUddW
000
JJ
UUUE -C
g
0L
O0
Y.
WWW
0
E >
~ Ui
W.J
J6oJ'.o4-
t0I
I CL
J 0
U.W
LU
aIW
o
Xrr octtZ
C
C
W
*Z
n
75
E8
j 0 5 Wwu~L
W -OWGU
*3U
~~~3
tOOOOO>>
3i
o ->0UUUUUWW
o 0
J J
U
W
i
04
w-
z - .
bLb0SIZ §{Bt8°
.J4gJ
a U(v.Wi
W j
Z
U
Wlt
5W
6002I-
" W
J s
UV
-
oocoo
$X
C
O.W
1m,U
HRWWvIm
055U
$E: (OD
t 0N0
t)tt-C
0
uuuur
WW
N
N 0 -t POC
000000DN
UUU
MCuuu
(4
-a K
In
N CI N
H)
0 % -1 N a'
Nr D0. 0 N)
II
Z
C
Ir
1(
I No
0 n DIn
-j
Ni
0CD
-j
5 tL,
F MD
o L
I:0
U)Omm
m1+ce
n
I I %DN
&N N N5 -C OWN O I
6*_M$N_
h9DN
DV
I
Oh Q
N N
N rI(g
N " N
vq
w 4
t
0
W-M
RR
-NfisNN
mxD~~~~n-'RNWNCD t _
N
NM s N
NN@
N to 0 DRN 0n
sF
N
IJJ U LL N
t-LL
00
-N
_,
0WI0
--N--i--lNNN Now% tW
I
II
I
IS
I
I
I
I
N
W
jr
t
I
N
-NI
N
%tD--lwi0
%4D
@ W9~' -N
00
I-')W M
-0
Q
O~w~WN0DD
rN
I
I
I
I
I
II
I
Ia
m
0000000000o00Lou00uU00oououoo
C, w
. B EI8
0o
Z
ILV
-A
w
,
~
-s Z.O~~c
QL e L
W W LLW W
W
5
DiLU
0
Q
QQ
UOO t OOI
UUUo0
cc L
ZZ
WW
-
N
ZZ
C
t1.ILLIL
StCDoF
=1zz0-Fn~t
>.
i
zz
P
S
5WW
c ^
w U
LWW
U
I I tflI-IIIJW
OW~Szfx
U
L
YO~UUD£OU
UXl
tW
O
f
^WWUUWUUU
t
1,1
LWWiUIOO>ZJLW,&l6
Jx>x~3sis
w
Hwg~~~·Q~
Hij ddjcra
wIxz~
w
of O
LLLQQ ZLL
%%
W w WLL LLL
LL
W w wJJ
J
IL
ULvY
~
J
Y11W P14i C
h
ae~
e
wUUU
MJ
76
* WI,
o ULL.
%D - (D - LL
X D. CM-
N
iM. 0.
*W.
-Nt_
N
rv
OWN
--
-4
-N
LL
tnrNMt
N
'
0
N
MNON
eNN nn
NNODW
O-nMaOW
W
trl
0
W-
UNWN*DNN 90
tt N N -
IO WD
N-OVONtOIT
C\1
N
) N 0C
ONOtD
vWNO s an
N VM
KF
OnsNpiNO2__Sm
t)
N0
(DD
t
NN
D 0rw
t
O
N N D
-PDCDSQ
NNN90PO
N OD
N
N'.NNN-NNNN'
(~ 4 44 94 4 4 14
.
<
cr
N
N W N N
-
-
!ola
UUUOU
LLt
I
EF~~~U
8
0.
8
q:
I
1
§
Z
asi -- EXlLVu
w
ai
L- d
Cc
-
DIt
S
v
wO'-Z
JW- uI
-~X
o Bo
Eo
s:"-
cAJCn
Z1C
X_L A ZC
I
Y
W
LF1
o
-mitHH
U
Ie V I
48L)
Cc
O
LA. O.Joe-i
0.Z
. . 00 0
0UU
WW
I I -IlA 0.0
d Id
WW
.J0.
J
06 aZ
-
N
IO
CL L (L
W W. .JL
W.J
LO W
JWjawwwjw
J0
OQOxQO
3-
IdW
IL
Www--
-ww
-
LUIft
m9
.J -J J 0. .
0
J
-4-J
-j-j-j
I
5£
-O4t
OWWWWW
'-4
0
"
ow
3
0
-4
b'-4
04
77
ww
*
WI
, LL
I',
a.N
Lii)
Q
- U
mm
X
X@
;f SX
N
-MhOIt1
No
0
MUMHOSU>OOM
NNr)tQ~nD'-4)Vm
ONN
K ~tC
TNMO
000u0a9No
aN-ntrNO
lNO
rN W O-
1OOO1N
t
_4
Xt~-NXtX
OOOMM
UIL
0-UCJ
NM
_ YQC/DN
M
I
OQIICDr
"n-SNOOOM_
ON
hO h-4
SLc-DICI
lDhGolnt
1W -1
Io0
U..
CM
UUUUUCuOi
u
UOOUUUUUUUU
YL
W~~~
^ CZ
W
LIM
OW
O
>
0
-O
CW-W
Zr
W"
Ew-b~
WLLIW I j
JOZ
J UY WH9
twL--Oa:
atWWmjUWrm
9Ui
UU
Ow
Ln
U I iL LAE
I-U~LC
3 X1,
J Z J J1U
z
42
u
a
LII
.Z
MWWWWWWWWD-
ctat L LLl,1i eJ
I
0
I
L
cccgrr
B
UUUUUUUU
UUW
o b ig
Zr ig6
g
ZZ
Y 0
W
W
-----
ZWC
W
coo
Co
(L z z
4UH_
0$0
JLZ
78
U.
z
MU
VIePIP
Ju0
a.aWz
z
'- ZZ.oo~o~~zz
.
we
.
ULL
C
toj1-
W'
wrOvmvvmNmvmXn ^ m ^ ^ VU
-*Uw-N
t--w
WDNOMlDU -M-NX
mIa coW.
U IL
*"U.
N
N
- D-
--WO
NM
-qON
4Q)_O--)Fe
_
hm
-
1
N
NN OM
a
N
- N
i-N(D
O-tDM) It
t N
4Ca
_
P4$N K)K)N NNNN
ll a^lLVNONONNU
tNOs-OMN-Ono
0
NQ
O NO-NNnN
N-
K) K)o) N N x)
N N xi N uuuuuu
N ) 1 W7pi I) N
uuuuuuuuuuu
K)
UUOCUU(U.UUUUUC
0
Ql
D,
0'-1C0
0(MEJTH| is
UU
UC
U
m
iIf E Zcr
_ _ _ _ _
zz
U
> m
JWjWJW
02l _ 0000g.2On
- o0
"
"U
.
UU
W
z z*-otnoww"6
iLI 1.-.0 A0
c
Z
W
ZZ
Z
usm
YUK
u~m
fE
W
$ ZLL
ZW Zot
Jm
u SZ
Ho:Fu-w
-4I
O W I
WJ
J
UoRnwulr-so tY-_H,>-l%-MrT
. . .WW
uuULW L_
In~moC
WW
U
W WWW
JJj
C~t*Xt
.
-4
P1~~~~l
LtI31
e~g
oc
.-4
zD
£z:
VIt 0n c z
~i~uUYYYYYJ~i
b-4b.4-4mmcWWLWWwOO
mmuww
WW
c
vvuvvjj8~-~
z
1.
-J
79
le
I.
0
W
.UU.
0iU
,z
OD
CD
XgQc k
N
MVN-2080NGMNN
I=m
om
WL
0.
0
NNOni swaon
WmrtWmWWWmNmor
NWCD
N m w
OD(
-Q
N 11 %
-N
W)
C
N
N
%04N n
66 1) III ----D1.c-d
W..
(LO
.
.
UUU
.
.
1
N
('4
V) N 1-1
D-
mcNcNNNm
N )N
N
.DN W
I
N N
N N
n
N -4c
I
IDM N N
I I
I I
I
000UUUOU000
OOUOOOUO)UU
Na
LU
NJ
0.
z
inix
O
W
.
Q-WWI:i
L-t
r 0-I
0WU30 CK
wr
0
UW
mu
Li
UJYC
OZ~
"D rWz
>WWC=CMMMM
i
E
U
E
A A m %J
( ZIXDWO .1
J
WIJW
z
U
wQ
UC
m ¢D
iU,
Li
5CLLJ
-.4 M
>
LI1LI CD
U
oLUO
O&WW333ZW
JOOOOU
g0.0.0.0.
0 O .J
ZZ
O. L LI-..g
3£.
00.J
ZWW 0...0E
tj*IIn J
WW0.
1---c
Y
WOO..
s wsn~-
0
WWWWW
WWWWWW
ad .a dLuIu
DCDL
0CD(DC
ZLA
oC
DOD
ZZOU
wwwa: n668
QOOC(COOOWU
WLWwi
mmxm~uu EZZZm3
a
MM
l
>r
:
r
80
o:
MWOOOOO
Jmmm
mCDC
L
*
*s
U.
C.8i.
*0%
*0
:SU.
*.
*
*
InCD
A11
'
N D - N U) N %Jt
N N tV *
N
N
DV)
N-- M I tD
-
9
V0%
N-ItIL".)0~0
61 0%
0%NtrD)lNI'
N'.) 0N tDIO
n
I
C-
W
M
10 tO
N
N
--
1N)NNNNNN NNWN
NN
NON
N*WNNn
N
N
M
N
N
W
IN
UCL
~~N
~D%DTN-P-hD
& IOD V
J
0 Q
tD ID
)
m
1
8~~~~~~~
P
W CDN N f9DC
N V) 1 m
N CD--D6M N
N
.- t
IWgI)Dh~r
fIn N
-
00N
N
t.
N*NNNN
L
m
o
OUUUOUU
Suuuu~uuuouuo¢>uuu
o2
iLJL
O tO
UV1WW
.U.i'-W
~
j
cW-
Lw
^ I I
U
Z
U
a
w
U
I (C zz
OE~C
_
W
J
VI
IL
XY i fU1
Co-
z, 0F
iiw
ImW
-.j- Kim
C>M
O
1
COLU
O
U
! U3YL)L}~~~~~~c
CY=
IxOU@
~JCJJ
LQX
III-Fdw
00
000 cooJaslwz
WInJ0..
.J
~
-JZwZw
LggLUUUUUUUFX
OOOaOOOOO
0
wwWWWWWWW
g
w
I z 8~~OH
f&&&&&&&&
i~z
LW
zE
R"
U
'!uW
E
I-. t8
J-J
-4
81
a. 0 0 0 0
n
W- Z i i Z t
D
m
CL
U
C
DO
D.WU
-N......t
0
I
o0L-(DLL
.ULLW
TCo>o
IX
0.0
in m
.
a
tB-
~~IC
WDN
nt-61NOD
MWWO-1
N-*
N
NNNOnNNNWWNNN
-S t
WDN¢llMNt--0-M61
N
om
N
C0N 6 D
WL *
¢)0In
Ir
0..
~~~~
OUOls
-i
U
>~~I
U~
E
$N1 M
CDW)-
N Mt
JL
-V
. . ..w . Wt8M
N N
N
O
VI
WW
WZ(J~~~I-ILL.
ou
V -O
NNNN
O
I-WW WO
iJW
O
(D-w6
0
~'I-0C.
H- r.OtZ
ZJ FJU
u~t0.
Ha
-jis"P
Y F I .dw
OO0
3 3fW0EZCCI0.0.)
v NJaaa
v* JgrDDUCH
LIIC
1:LEmnaduuuuuc
0033AULLLX£::FUUUO
Z ZZZ
LA
Si~~~
Z
Z
H
lY
w~~~~
O'OO
nmoN
N @N
N-SN
0: t t 0 6> n
aw~~~~~~..... . . ....
. .
QaC
Irgq SRV
ONNVJNN
N
IN.
JD
mL
1C I
DNN
N-D
N T
u
N
L
O
.U
IZa
W
uAYO
LA
A0
$I) a
w
Z
auz
O->*
0~~~~~L
.0 .(
-EXXX
CC C
QUz
JJ
WWWWW
0X0 ~~~~wTQQ~J
L
z
-WF:~~-eulwwwwooo
cK X-imozaron
mmo - -- 0.
Oulwozzmmmzoa1
-zzHz
*m
'm^
n Vio
%n
z= o o
W1
U1
O m aU o
^y
yy
UEwW O
mm:
D
Jzzz
L
W-EE
£
0$U
~~~~~~~~~~~~~E
.
ccT T.T
£~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
=
Z
82
UU U
.
W
'WI
.
0 G
LL
%D Oi .
a. 0
i
"4
I-
t
N
N
r(
~0
Ic
N~Nmool
WNN
NNNONNtm$O~mW
ONO-o
-
0 tN
fY-
"4
OK
r ^rnt<D
OV) %D
N
"4
WNWf'
l0%wt&RD1N
OMNOTMNOOMWOO
(. (UL)OUDNWNO
(S6
@ ; K@vi(M1~t4
stNO8NN-IONO-0
NVw1nON-000WOM
W
_: !DK
I
.
v) Z
I I I
I I I
ODW
0
N
o
I
0
o
uU0L)OOO
ouooouoooouo
00
00
0
Y0%
z
Y
a
w
OwW
m
ORI J
au
J
i;Z
T
Z z
o
-C
Z
4W
J uU0O"Z
3"I
tuA
4iFU
:WWI
J
P fEjE12iME
a
w
w
'ff'o
wu a a
wwur!%0~7~
QW
0
W
ad
>OU
O
I
0 U UU;ZZtZZZZ
aZ
I
8~wwwwwww
Touso
c
m muuu~uu
U0U
Ua
0
u 0 0 0o0 0 0o
z
ZUlY
88.W
ZJ 3Oi2Jmu
IWO
!i6
~
w~x
QLLQOLQ~PL
-LCLM(LL.0L
!I
zz Sar
o
>
wu_??~~aW66666
CKj J ujLU CKC
)-I
C
8I I u,
U W
1 a
W
zZ
83
LI I IS
lW
WU
UI
.0.00.0.0.0.0
T UL
Om
om
O
W
Z
-nWI
I I C I If
"'--
1
Ii
I I
xL-2 W
I I fIn I
Mt-UfNN
D
^-
I
V N -
^SN
N
c ::gl:cU~l^GrL
a
oz8~~9~~M~~+IU9~c\
zc~CL
~DQIQ~aD2
L uLLuq
W uyuuwmW
t4ilZIo
a000
LI
D
D
w
4>Xt:m
FF
0Zi@0000mw
UUUUUUUUUU"JJJJZZZZZZZZzooQmm
84
466
0
Wc~a
(a Cj LU.a
0~
IF @-
-
CD
U.
tM--N
N
a
in
LO N
La
L<to
IW
o r--N
bm J J
w
Lf)?nc~
W
I
cr
1J Jooso
J
W W
x Z !-jm
- -I
N.W
-jW
9-z zLfl
Z
00NOu-uzzz
I.
WOxuu
OWWJJ~jj
OJ
wwwwo
IzL
O
RR
Jo~~~~~~
U
U
rVI~~~~n
-j
9-d
WD
(JOIO
NO
W
COR zz
W-t
4
Wwwncwwl1W
occoac
g
JU
iW
jjjo
W
Wx-J00
WUtW
0~e
14"14IJW
Egg~6e3"0000C000W8I
un:0.
LUUUUUQQUUsUH66HU6UUOUQUL}
4000nUUOUU
ooo o
U
U~UW;;UOUFZZZZZ
OU~~llJJJJ
OO~bJJU~nu~e~i88
LJOOY
(is l .) UUYWWWWO
C. C) C) C)
W~~~~~~~~~W
J
O0U
~~
85
aV
UDD
IOWAA
LLU
Flw
'''R
tINJ:f
Y
|
d 0 we.
X:
-
at -
I-
N
to
n
N
N
N
nOsWDNonN
-NMMW-N
VW0WMD(DS
DNNhW0(DE
u1Fm N Z nWJ6
1
om
WJ.
O m rN
W (La
444444444
UoIL
N-6O
-O-Nn(~0
S~~rCSVC~QU
N
33,6
mov.ItD
cOncONOO
O N TNn
0-0
no0-mon
NNM
If--lN9')
NMeD@
nSN
-WNEMWD
m
QMN
M
N
+^t
*-
... eai
441~4444
l)SYQ
o
NN
*
LUL
UU
UUU(J(.
. UUUUOU
r.
W ibt4i
"OfLL
"i
W
WL
NN
gig
6*66S
01
Z
O SO
tS1rh
--
mm o"Sn§
X@Xt
(JJUUUUUU(
%DN%
Xn@n
o
WV
DtNMOOWD
DnN
.LJ..lWJ..J
J
Y
II
tZWzz
4
y O
-Y1
E
X
0.jti
00
J
zz ww
Bi6
ww M0. ii W.
lwpd lGU sm
W
W3-@~
YW60
OWU:3 00ww
J60
Y V
W
N000
J 3: JJ
00
3W
W
O'Va~~~~
Y U wlC,
UI^J-t~~~lllS
U U JJJluX§
U *
MU
Z
Zu1!
_V
U1
P1~~
_ 0-(U:
J d 0 ltssRSa ~ CU UiSQ
U
et
W
(LlW
wvl~~ WwQ(IQ:
Y
U ~
>.ww
W~~
3I
U
W in
e% t B
L:
LY~ Q:
r
P
n
w
_
w
86
W CI1P
L
0OR T. Z_
0 I0
X( T
WLO
I
N
to
UU.
I>
I
In1
N
In *
*
N
om
OL
-4Ntt
W)U Q0AIn N
W %O
NN-4
g8§nN
T)NQ
VI -
0.0
N
NN
-*N-NtrNV-
-M1t
In6
I W-'@
M-OOXNt
N
.O
Mot
Z
r
C~QrrUQ~M
l=-l
-N-
N
llNOMOT
O
NOW .-*
NO-ONTN MVN
VW, *
0
in
W.
NAONOMVn
N
D
TVVthSS S
(DU
I
@t
SS
0%
@
N
00
U
Ir-n-nLn~Grn
LLt
-
9 N
t
UuuUO
JUUOuU¢DLOuOUOUUU
O
Ya
N
OS
~uz
ZCL~
0.
0
-(Z
yw
j 9 2s
^o:Y
Io
FSX tHW
&a
0W
>z
W
a
.
~
I
-j
I VI
Lq
J
m
0
ui!i
0OLsUC2US
HU^fi"E3
WVLL
YOI
H1 IO
J
ru
PIwlfw8
~uuuoouuu
IU
@@FFFFF
WUwwUwwUUUUUzz
U
wJJ-iiiiaJ3
jjjjjjww
www
z
C" ZJZ
i
owaaO
OJJJJJJJ
J
J
JJJWWWW
ZGG
Ol~dddddd0
W
WWWJ.J..J.J..I.JW
50
&n
WWWWWWLL
W O(LL0ll(L..
tLG5
f~ZZZZZZZZZZZZZZJ J J-J 1
'
IL0.0.0.0.0.O..0.0.w
. 0.0.0.0.
fX
W
P I
a
87
N
N
c;
R -LL
0x ?,, I
8
80i
-Vdt
W LL
.
+nL
UZ
U
V,0-
- -
N
n8mn A V~
t
tFoN
V)NNN
CD
W
'NIi
% I NtON-O% DD-N
0 N N tS
4D-
U4N
N
-4D
i
-4~
SI
I
I
C)I
I
i
10
S
I. . . N
I
V *INN
-0
N
t~NNt
MPONffHNI
- .. NCD
_4L
N _
tCD^D@0ainaSS
OIJ.
A';i,$
N
-~D
N
0
M.-fN0 0
(Dv~\o-
NNNN
uUUJUUU(UUUU(
isI !C
d I mJ
i
%k
I i
5.. E mm
I
U8Z
3"o J -o!iK
Y ~
~IYfnoclB
cr~ti;iY
tr
t~idst~tI
I ig JI-Mjj
(k
nz
,,UQUL)QUQQQU
W~rWWWW
WI
wwwww
U
W.
.
mrwerwvsoon
(L
fL
%,
CL0
a.LLLwow o.
niiLnd
.
ozwu~f88f8888800~FF
CE Z
&wu5
EE3333333335333
3
88
I
Hh gows
0On~H
. L
2
APPENDIX
C:
Output of Seasonal Gas Substitution Model
GuideColumn:
( 1)
State where plant is located
(2)
Company name
(3)
Plant name
(4)
Coal displaced by gas substitution
tons
(5)
Cumulative coal displacement, thousands of tons
(6)
Oil displaced by
gas substitution
at plant.
at plant.
thousands
of
thousands
of
barrels
(7)
Cumulative oil displacement, thousands of barrels
(8)
Gas/coal
calculated
or
price
gas/oil
differential
at
each
plant,
from gas prices in Table 4 less coal and oil
prices in Appendix A.
(9)
Billions of btus of coal or oil displaced, or conversely, gas
substituted at each plant
(10)
Cumulative gas substituted, millions of cubic feet
(11)
Cumulative emissions of S02removed. millions of tons
Reduction in deposition of S04 at Adirondack receptor as a
(12)
result of gas substitution at each plant, kg S04 ha- y(13)
in
Cumulative reduction
sulfate
deposition at
Adirondack
receptor, kg S04 ha- y- 1
(14)
Percent
change
in
sulfate
(S04) deposition as measured
from 27.5 kg S04 ha- 1 base
(13)
Cost in millions of dollars per kg S04 ha - 1 reduced for
(16)
17)
each plant, i.e. the marginal
cost with
deposition of seasonal gas substitution
Total cost in millions of dollars for gas substitution
Cumulative cost in millions of dollars
89
respect
to
N 1~~~~~i
...
<1i
NII 0:"; 1! *~Ni U! N IID9! N:n4i 1 N
0!"; N II
@..*|@**e@*****|*****@...***@*.
r
- I IltN
|
I
s^@"
N
%1
U I0
i
I
| |
AI
| | 8
I~
! II ONNZ
I I~ I I I' I-I
rvg
t
oI I NN
I' I I NI! I
I I I I
NC.t
n> NgONT
I I ItNN-N
I~ I~ I II
III
II
WSP~i:
S-a1 .I M.
I
CY @
Ee.
I
t
iii
Its
CY~3ddi~
11111111111111111111
r
ago
II
III
1
II
S@001000~SII0000
00000000000
I
@^z
I-
U UZ%&UeLWWWL#I..U.U.
MZ*@
0
= MZZLL=ZZZ
l>^>sE0t0003**z05gGO33S333d~o3k
9h
@
@@e@
6i
@a@@
OOOg
@@@@
b--a.
33
iaiii
e@.....................................@@
e|S~i.5,s.#a
tt@
r
IZZZ
lB
aj
§,S
SI...
Z
CC
r sie
85eii@
OOOOOOOOBO00000000000000000000000
0000@0000@000000000000000000000000
I
c
s tS!| §Sila6g
w~~s
isllZitl~t
W.J
j
j § i l lMl.
1lN
31i all0fl
CY
·is~
rceca
~
0WX~~U
cr~ a
,,,,"
^
Bi
~ cuWFX;3|60BBS%
S %8
~ rXrM
Pi
A.g8X2E
.
LE!~9
.W000
BjizQ
%sXu
'£Q
! l
m.
srwrss~WJ;z
wa~zzzzzzzzzzzzz
PI
M1' NOORJB.J
=ILNOOIDO.iINIDN. D.-1B.
90
hf
~R~S35fi:r(~rrr~r~x
w D at 0
We MCD9 ia In
CD
a a CDM
a Ct
D ID Mao CD
D 0~RXR~i
. . . . . . . .
U4
. . . . *. * * . * * . . . . . . . . . . .
ci0 _, 01*,, a a a a DCDCDDC
a dl
CD
a a D 0-~
o wo aIa hi
CDD a CD
rA
hei
.
a a (M-
W
t
0~~~
e-v U
1D
.4
n @|@|^
NNV
f
|ss@||
|@||@
I
I I)Olsn~h
IDBS~t(
TCU(U
II
M*--8
2N
AN F
@@
@@@@@ t @@@0
~~ ~~~~~~NN
re~~~~~~~~~~~~~~~~~~
N
N
N^X rS0
NNk,
rr
n5
%
In CYCY
I
%
c~~~y)C
q~~~~n
CYQ~~~~~~~~~QD
nL
r-rr-r ~
000000@00@00000000@@000@@-@@@@@
.j
10 a
10~
%E
liII0
0
-jZo3.
Z.
0 ws
s
~ ~ I
Y
CYN
N3F~~~flZ^[-@ @ge-E&SDNNGRR-S0~~~~NN
-N
c-N
--M
-W^
f~l'---oo
5 gtX8NN@N~~r
E
tr hc~"hQ~~r)
tg3-2 - |s a5(2 11
ghN
S3E
CC
O
ooooooooooooooooooooooooooo
r~ j--W
_XN
fi
_et(
SMD)Q
4-Nb
-(
_66
@C
O~~'o
tY
* Li
N
u
vN
PiiIn~~~~~~~~~~C
@fr@ 2nlrT)b@
o@@
UEQ
0
01o
@@@
O
~X
@@@cnr
D^0
@ ui_@tt
2N
~O VIffMw
VI VI
Nt N .
11
1< 1'v 0
X
Nx
issss
IC~PBB(B~CIBMI
O
d
0
u
<s,<.,@°@X@@@@@@@@@°@@@@°@@h@@@@@
7
e
N
r
1; 0
O-N f-
oi
()P4
t0oNOroo=WNtM
1
1 Oz t
i
ct
ai
SA 8 gS ! ii
rQc Zo
OV
rl6JJ
Vs
_
E
rcf
r
.
2; NCW
s *Lus* O n v N @-O 2 1i1i It u s
O
o~T *~ NO O tM IN~~O
* IC
Rl
s0
14
@
N6jN
^N
N Ev
1D
@
1
^ovNN
o
s
rfi0 ,
n
0<0oN
0
Z
w
O ^
Z=O -
EEEEXE0z'SEER0xE0ozaizoS0zz1urEBES
91
04
4
N-
0
vW
14
s
v
10-01i iF
Oe
aM S |i
sH
WO
B.
DE
VV
I
Ig
*nI
I
-I
C
N
'U
L
N
0
,
e @-A.
k m N @ @N
INJ N I.NI N .IIN
ME
PN
-- ,
INo
I Iat
ID
.
.
CY
.
.
.
m,
aO
a- 1
. .M~QIwrcO
.
.
Ri5
1Dap1.@
DZD@I
.
.
.
.
.
.
.
la,S0UUUS00
WWN0S00
L)
-
NI\.'INWV
CD
WRN
.49
.N
U,;^ In8
--
M'U
t
!mS
S
4(P~~MSFI~B
--
--
---@-
-
N
t
=OS N
=~l~loN
ti' @-N-ft--S-S^YP)4ffi00SNSOWRESI>FS§;g@r>
C >13
e
nxe &e HHHOOMN
z v5ss
R
=
20D
OO
a!
a,
s
Sfi
-
80
0 IL
0.
I
IIs
A.1
si aiaNWILccQ
i>it; QI-.
tWUzffi!W1o#SXEX
D3Ji
92
W
_
;
N
'In
o -
-
-
-
#NkZYvi
ins
fv 0 "
Iah
I vv
ua
LiP,
fg
oe
o o
0ll
la
.
Z;
2
Ee
NN
N--i--N
It
i
o
u
Cy
in~tsoin
E%9
c(
F~; ~
I
-~
n~
2
CyN u CY
N
N
iS
Qrlt3
w
m I
rrgacl$?,,
Z
rIIG.EU1r
o~~~~o
93
~h~~
mmOftk
-M
orOTI
0f
NM040
Oa
mT
O"
ON
"Iir
tU .W.NNNNNNNNNNNNNNN
M
U
N
N
f
rl
.4
II)ISS~MF
@NONr
lDOXtfO@8OII40-<ll~t~lN@@-Olt
I
O~~~~~~
CYCyt
y
" t u
Y NCY
u
NCyN_
MNNN N NNNNN
L.LFCYKa.i
NNNNN
a
CY
...............................
NNNNNNNNNNNNNNNNNNNN""^"^""^
r(
& AC
CY
Nf 111D OfINl leNNNNOII
NNI
II
1E 1-U
0 rr~~n9
S 4A
rcc3
Y
a8a
o
in ~6
m†SI
z;gS
N_5
25-°i;
rrrc P5I ~rr
a~~fit~G~~~
o
oooo
~ oo ~l o o
nr
~
I 0 lC
1M
N NIFt
-- *NNo1R
o
ooo
octcrrmfin
~~~~~~~~~~~~
.
S
O
†~~~~~~~~N
sn
C
aY
8.
a
tBr:ffcu: M. eaz
~~riE£
=
rNStt3Bre ~8nrOYQD
IeZZ~L
6 I Q-J
W IQ
I&
I- J
jPY
~
~N~o~+NJ
~~n~~nd~d~~N~~onn
~
$I~
flt%A
I
lala
o
19"f f 3
VTUIMIM"MO¶8¶iA~r
VW Hgojw-1~~~g~~g~
=°UU IIVVTTTVVTVV
~SUs
W~~11~11~~~~l~~~~~
uu
c|c(il! iiiii
N
rISW
tA
J
-i
~c(r4
CYI
if
~ .j~~~~~~~~..
~ ~ a ~ ~ Ike ~
Swll
~VW ~ ~ ~
Ii-
Om
! ' -EE-I 1 K
ttui I
f
E I 9E !31'fz
i
94
19t 2Z II
c 9 =4 2c,.
z.f z I
U
a-ONaa
IF "O
IiI
S
.
oV
0
Q%%rV
NFN-DtF@
94-94i
kw
.
L5
Ieee
..... *
I
Sil
egg...
*...
CY
a I,
kNr-
in Ito
e@I~d iCVWUU9494
39 9494IYUIY
3QD
UISI
i
__AA~ff~M~
W~
_4A.94W~94W
94A. P)f,
tAg
8
nb
r
US
0
vv
n
ac,,~3~~
d0".S4
"ieBIR
s~sS;!~e>#s-32
M
F"W
In I
r;
MMFlnnnshhhhrrhhh~nnlfsssshhhhhklk
N
nS~~
%
8
,,
la94.aN..S~b"
l2l3 sea
33r
f!g
1
94V
o
em
to
egg.....
I
'il5-
0942
94.
-a>~o>>2>8%
J
w
a s-s1
~~~~~~~~~~~~~
Z
Oe r|r~
s ue
u r1E 31S i
P
o;AU
9494EV
""""..........
*A.
jnpigz
fQ
"4DI
WUt4VAD
%- Dl - D- % D0 . R.K..kk
.e..........................e.
"I
?J
sv
giE
Nfl
RO
m
O>s2s~02>~t~lr
ISIAl
OWS
oooolooooooddooooooooooo
ff3S
lf|s l9lFflVVVV
9
gillllS
ge
gg
VIVVTVlVV$i
VVs-
CY 91
4
994
r91f
a sis awl~I
zirE~
@^,,,igiltigM8
"" i+,,?
;I rfIlrccS
419
t
ccaE
~
~~~~~~Ae~~~~~~~~~~~~~e~~~.
i~ n~~r
'D
UIUhcWl
_g..
PN
-geleess8gge
ge.....egee....
1--p W88
S I
I
A
a 9a
-
j ~~1- 3I
3II
f~~lB"'~%~'?:
a
fi
iio
..JOPQI
U U 300 U000000OB
CY- TIti
inB%~t
HIK
Lit
Z s~~~~~wP
Z
iZP~~~PMmfl
01NE92i-0 fzm
tB~~~~~~H
95
it
c
age
tie"
I
"WI . t;
QNoZE
9 11i 6tl-99{, 0
)
U VY)Y949
m
N
o~~~S
_~~
_r
.Au%
CS
m
C
CI
u
I
: ^~a
ICZot * -N
3wN
~4 ~U~94
&rr
al
WUN
Cy4@@'
@~~.W
*94V.D
MON
99999
I
S
N
ffloNtNfNffiff
DtDNN
ll@g
et
S
1 *£Z222OP
N
%Oa94Inr
9NNN
CD94
-
_91¢
.>;fie-g
tt
IZ~t I
x ig 4
;>Nj
wjrnXX
swempss
essse
IA @@@0@000000@0@0@@
laI
*
Ok
0.0
*
I
°
I
r_
u
1,r
n
~-J
.rr 4
NtCY
;
9
;&
N
10
0
<
_4ini M
O~~~~~~~~~~~~~r1I)
N
N1
g a!mvs'2'"'
~~0S
~
'r,
r
0%CY F"t
°4
U,
,n
@
in
22e
g
o
V
0
j
E
9 WHelImemeesmemsmms.~msemsmws
trmhg
RlllCcn
meee-eep..es
"
-
N~g
l
I.-
*
K
IWN
a0Ii
n~
<
%A
S
W I 09L(
W<WO
0311
m
7
w w4Lo
0s
iE
IL 5
I'..S '.'s
iNO
445 CD % f
B.T4NSVS
O'
Nt
'a -Ia 0 OX
Na~N zfhiapB
BaJ~r ~~~~~~~~~~~
azm
0
oi@.4.
_N
R ~~N
*II*
~
~
~
in
~
m
~ g
~
IL
i
^
CY
a
ahiFL W e-ehi
@0
1 O.
C
96
"o P..Jhi.J94
3e
x
Q
-I
=
I
u
N
*;dt
liN
mu.
gt
Vhhh
rhh.Nhr~NiVziFiU~
*I
ID*NfiJ-0-O5
8 _aggiNgl*NZi~llNNt~l0)-0F
(U~~~~~~~~~c
(O
rl
g gje
gag
s
la
0>!~ ~~~ I|v-A!
0@Wg0hli>s30e
S
eVIS*
SIC
1Si0i
I-
N
I
S
ineAARRnMMnnnnnpRnnpnnnxpppRERER
nn
IL r
la
A"
WSYEK
11W
UEI t I CIV
J~la
N
~~~~N~NN
~
nnn~n~nn~~~~t~~t~~~Pty
*U
WWt~NN
!|~4
-o SS
fW
Ea3;131iB
~AAf~g
il~
Po9
Nt|[h~uBM
NI
jIjs t re .3fII%Q9
| | 5kigi
TI 0 .
I
@ 0In
I
5
0 in i i
0n
o!!ifi|fi
0 0 0 0
0 0Win 0
F 00-
B
-,f~
nna
alle@@@§@e@@@@@@§@@@8B@@@@@|@
jele
~~UICI
NNNNN NV&r~psnh
h~
a
as A
%
g 3""§Ng2
Ma
_ , In
t
l N
i>§f
aw%.m
CYU'~A
0000000t
LSt
IL
t
a In CyCY
S
90006
cmCy cm
s CYeV 3 "
N
000u000
P,
CYCyIn N N CyN Cyt
r5
oi~~~~~~W
@-z
~Nsx~ll>8#@iksl"
cY~
t]~~~R1BS~~~9J~~ze
|"ass~0|s
~~BCY
C2ft~f
CYm
vi1o
akr
-DusC"r
Ne~
ieBr~lli3
ACS
N
A
pot0|@N~a-
z
g^|"
"fi~
f-00-rNNaN"NW- NN"NNNNN"
4
lN N6NNN
@000000000000000600000z1000000000
mjua.Jdj
-J
e03i6t aDttltoS
mBi
8S>|iI2igag
e o~~
0.0 UP3^_NNNNNNNNN--N@NNNPNNV3N
_s
-a
s r,
Su ..
aSUSSeS
Nf t~wS~8f- -' - PW
"P~~~~~~~~J~
"I U,
" ",~
~
0
KtO
5 *~ Or
S.U (w~YV
alw~
.Ji~0
0~~Jb
0.U
--SwaHs
s. S
-~
e
U
I5
3a
~P~SBI~P~P~~~X~,,,~t
~ZZ~~K1A
97
z
t
8CD
4
tJ
a
CIn
U
m a zaW
.11
9WZJZW.~~~~~
S
yt
;S
i ge
To -ID-4
ON
ON
I
w1 la
0
a
N
o
t
a~~aD
U tii
,,vi$
"
-£
N
%i r
E
6
i
A% - Mu M
N
t
'80
47
0..
........
y.
NP9
***.
a SVO
n
-
U0 M
won14UUU
De@ 0.
0
E%~~~~~~~~ia
~~W!19i
1i
@-ue
N-| M
00
.,I
CY CY
t CY CY
U
4444444444'99999999NI
NN
·Bs94
RRRRXiRR-Rt~t>;ig{#%>5RRRRRRR£Rgll
.
. ...N. . .
946 . '. . . . . . . .
IL
4A~
N
M
r~~~rV
--
oW^^o~oloo^^o6F---------oo-
~~~~~~~~
~ ~
~
^P
_
~
~ ~ ~INo1CY
~ 0
14.4
f
g
Z~~~~~
6940
5%94
0'9449
>>
@
~
$
g
0'~3III9494
r.
~
¢ M
M O
I fglg80g5^
000*_"
o 1606669~~~~~~.4,
N
0
~
8fi
N
N
~c
f94
~
i
"^
EJNlHS
II~~~~~LII~~
N
NCY
.~~4
4
n
ZJ
%94
nu
u
__^
uunuuuuuu
i~~~I~~p
3
l
i
~rw
oZR
W
rlar
0;
I
S
"994P
U3 9J0.VN
X~~~~t
,, _P90 ttdt
OGo
i 8vt
U
U 06
VI %A
Lurn
UU5
U
M9
P-
Ia
!E
St
2ll9 II -i
9d
E
w w J DC
JW
W w X
hnrrr
uru
(%JNNNN999599
___
B
4~~~~~~~~zP
sl~~at*P
ifsf
8I , I M
.- ,I
-i
-9
X
9
NNNNN
OO
NNNNNNNNNNNNNNN
~~~~~~~~~~.94
k = zl l
l
O^
)NNNNNNP"N946IVN9NNPNNNNNNNNNNNN~nVmNN
nOP0 99 99~
O
w
O
.....
3 ......t
.6................
U'
d
1
0-i
r.
98
.
0
J
W 'a
. 911kill6
aU,9 12
A Mi
Witt~
,
N--N00
"4Vv"4U-^-NN@~NN"4"
vC0--'
PI -
-
so
U
"ti i Wiii"i
SIU
"4
BNaNNNNNn
z
.4
.4
4
.4
.. 4
NNn
..4
.4.4
.4
*;
.4
.4
.4
.4
NIN
NINN
.4
IL
iii!
xjslil:"li'
"1
si
NNNC4JNN
N
a "Wm
3NU
a
e
ievie
4 m-,sb
le
4 .4
4.
M4N
.
.*. I
.4 .
NrN
.4
.4
.4
ij
"k" N
nil"
"M-r
N! a**uu
sui
mNNNNNNNNNNNNNNNNNNNNN
u*
-z3rc*)~r orrrrrrr
,r
*J-frrrr
MUM
NY
"4 a
-
"
4
**
, zrrrRrirrcr
CY P
N1%
N
"4
U...
%
"4"4
I
e
I-OwC~~C~~~~UUOwE
I-IM8l
-IUU
U
U
j
W C
Q~~t~rr~Lr~I~ZtII~~~LY-
99
Ngi
on
--- o mew-i----
U
- amo--NO
io--i_
i
4o
n l ll lllf
§ |~llll
-iiiii
ii
ii i ii
394
iMMn
M
Cy
NNNN
6l Ela!llllll
S
l
NN
in
i
M *i
MM
NNNN
j
CY
VCPN
U".
J
O
_
~~MI
'I~
w
-L
>>ai
=
Z
0 oWt
gs
SZIzzao
- ,. s
~~s
Uos.J
=cz§=#)Jo~cisi
n
r°
100
94BI9
.
f ~_ _^W
^o^n)@
- 8 o\ -wXre
n"dX@@v_@00Nu%i"'
ctc~~~
rec~c,
h
tW
0~~~~4"
0
. -A0
la
r
N a
la k
m-- m-O
--
N
-$s
- -i----g
-----
X
-------
VIcy
PPOP
^E-wa
s .s .U1PQEgex~g
.s .. U
U
.....
-.....
~t-14ZI~NN~iO-'10I-
lr)N
.H
5 ~~~'"'"
N; ~ 2
Wfil"I"I
n o3
*l
CY
@@@@
Is
I
s
2
~
-
.
.
.
"'
.
L
tNM0ON 1 11
_. ~
. .. . .
. . .
0O
NNNNP-NuNNNNNNNNN
. .
C~~~~~~~~~~~~~~4C
-U-) n---rr)----W
cus~Bkere&^16a:r'zn^^
*-soiftxorii:
tS$ x
Vfl Wi 60
98H
MVe.
V
W W
eizs~~aiJeSIC""eglEI
R
fw~I~inI.EW.-O.u
. 0i3
U.
N-200010
00X"0^^00
0-- NI
.SI&.S
^--.bSib.
re U vr
X SJ
CY
|~~~~~~
'
E.
rgel:e .3
tK-U
..isK
J
reoglil;
Us
z
5.
S~F
1'
X
A
J
O
= dS
=
5. U
101
5.05.4'
5.4 M
-Wi
d