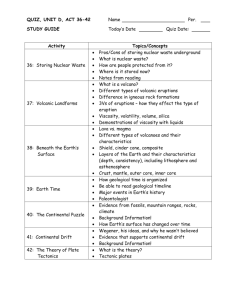
GEOS 470R/570R Volcanology L02, 16 January 2015 Handing out
advertisement

GEOS 470R/570R Volcanology L02, 16 January 2015 Handing out Copies of slides Review of minerals One-page questionnaire “Write it on your heart that every day is the best day of the year.” --Ralph Waldo Emerson Who are you? Why are you here? Class Major E-mail Questionnaire Photo courtesy of M. D. Barton “Did you arrive with a particular career in mind?” “Goodness, no. I not only didn’t feel prepared, I had no idea what course of study I should follow. . . . You needed some language credits and some math credits; some science. So your registration sheet filled up with required areas pretty fast. “I took a geology course and absolutely adored it, and I really thought, gosh, maybe that’s what I should study. But I ended up majoring in economics.” --Who said this? “Did you arrive with a particular career in mind?” “Goodness, no. I not only didn’t feel prepared, I had no idea what course of study I should follow. . . . You needed some language credits and some math credits; some science. So your registration sheet filled up with required areas pretty fast. “I took a geology course and absolutely adored it, and I really thought, gosh, maybe that’s what I should study. But I ended up majoring in economics.” --Sandra Day O’Connor, Stanford BA ’50, JD ’52 Interview in Stanford magazine, 2006 Readings from textbook For L02 from Lockwood and Hazlett (2010) Volcanoes—Global Perspectives Chapters 3 and 4 For L03 from Lockwood and Hazlett (2010) Volcanoes—Global Perspectives Chapter 3 Assigned reading For today None First assignment due 26 January 2015 Hildreth (1981) Last time: The volcanic center Course overview Tectonic settings of volcanism Definitions Igneous and volcanic materials Lavas and pyroclastic rocks Pyroclastic depositional processes Volcano Volcanic landforms The volcanic center Summary: The volcanic center Course is designed to provide perspectives on Volcanologic processes and active volcanoes Working with partially eroded, altered, and deformed volcanic rocks Applications to petrology, mineral resources, extraterrestrial volcanism, hazards, climate change, geothermal energy Volume of volcanism: ridges > arc > intraplate settings Igneous materials: melt, magma, lava, pyroclast Flows (coherent mass movements): lava flows, pyroclastic flows Pyroclastic falls, flows, and surges Lahars: volcanic debris, transitional, and hyperconcentrated flows Shapes and main types of volcanoes mainly reflect Lava composition or chemistry (viscosity) and eruptive style The volcanic center: fundamental mapping and stratigraphic unit Volcanic stratigraphy depends on Geologic mapping, chemical characterization, radiometric dating Next time: Physical and chemical properties of magmas Lecture 02: Physical and chemical properties of magmas Time, length, area, volume, and energy scales Chemical and mineralogical characterization of volcanic rocks Physical properties Temperature T° Viscosity η Density ρ Thermal conductivity k Crystallization rates Time scales Quenching of a pyroclast during ejection from a vent 10-7 – 10-6 yr (seconds) Cooling of a single lava flow 10-2 – 100 yr (weeks to months) Lifetime of single cinder cone (crystallization of small gabbroic stock) 100 – 101 yr (a few years) Lifetime of a composite volcano (crystallization of a dioritic intrusive complex) 105 – 106 yr (~500 ka) Lifetime of silicic caldera complex (crystallization of large granitic composite pluton) 105 – 106 yr Lifetime of a volcanic field 107 yr (10 m.y.) Length scales Diffusion distance for components near interface of growing crystal or bubble 10-4 – 10-2 m (millimeters or less) Height of a composite volcano (stratovolcano) 103 – 104 m (1 – 3 km) Height of a volcanic plume 104 – 105 m (10 - 40 km) Area scales Area occupied by a typical rhyolite dome 100 - 101 km2 Area occupied by a composite volcano ~102 km2 Area occupied by silicic caldera 101 - 103.5 km2 (25 - 2500 km2) Area of plinian fall deposits at 1-m isopach 101 - 104 km2 Area of flood basalt provinces 105 – 106.5 km2 (160,000 – 3,000,000 km2) Volume scales and frequencies Sizes of eruptions and their frequency anywhere on Earth Fisher et al., 1997, Fig. 2-5 Eruptive volumes DRE = dense-rock equivalent Vdre ≈ 0.6 V for tephra Vdre ≈ V for lavas Volumes (DRE) for eruptions of the last century Katmai-Novarupta, AK Pinatubo, Philippines Mount St. Helens, WA June 1912 June 1991 May 1980 13 km3 5 km3 0.5 km3 Comparison Huckleberry Ridge, Yellowstone 2.0 Ma Bishop Tuff, Long Valley, CA 0.7 Ma 2500 600 km3 km3 Hildreth, 1981; Wohletz and Heiken, 1992; Wolfe and Hoblitt, 1996 Eruptive volumes Volumes (DRE) of erupted magma Historic, prehistoric, and Pleistocene eruptions Basalts in gray All were explosive except for Laki, Lanzarote, and Nyiragongo Schmincke, 2004, Fig. 4.17 Energy released in an eruption Heat (main component for Hawaiian eruptions) Radiation of heat Conduction away from surface by convecting air Conduction into surrounding rocks Transport outward by gases Explosive energy (main component for Krakatau) Earthquakes Energy scales Press and Siever, 2001, 18.11 Magma Completely or partly molten natural substance that, on cooling, solidifies as a crystalline or glassy igneous rock Melt ± crystals ± vapor Constituents of magma Liquid Generally silicate: modified Si - O framework Rarely carbonate, sulfur, etc. Lacks long-range periodicity and symmetry (as in crystalline solids) but has short-range order Solid Crystals, glass Phenocrysts, microphenocrysts, microlites Gas Dissolved Exsolved separate phase Definitions Phyric Contains phenocrysts Aphyric Lacks phenocrysts Vitrophyric Contains phenocrysts in a glassy matrix Le Maitre, 2002, Table 2.1 Role of elements in silicate liquids Si, Al Network formers (strong bonds with O) Fe, Mg, Ti, others Network modifiers Alkalis: Na, K, Rb, Cs Network formers in peraluminous and metaluminous melts Network modifiers in peralkaline rocks Volatiles: H2O, F, Cl Network modifiers We will see these groupings reflected in classification schemes for rocks Silica content Ultramafic <45 wt% SiO2 Mafic 45 - ~ 52 wt% SiO2 Intermediate ~52- ~63 wt% SiO2 Silicic >~63 wt% SiO2 Some prefer to use a higher division between intermediate and silicic rocks (e.g., 65 to 68), instead of 63 wt% SiO2 Analogy with crystalline solids Increasing polymerization from Orthosilicates—isolated Si - O tetrahedra Single chain structures Double chain structures Sheet structures Framework structures Melts can also display varying degrees of polymerization of Si - O tetrahedra Review of petrology Rogers and Hawkesworth, 2000, Fig. 2 Classification of volcanic rocks by modal phenocryst content Q-A-F-P diagram Quartz (Q) Alkali feldspar (A) Feldspathoid (F) Plagioclase (P) What is a limitation on the usefulness of this classification scheme? Wohletz and Heiken, 1992, Fig. 1.3 Chemical classification of volcanic rocks TAS (total alkalis vs. silica) diagram Rogers and Hawkesworth, 2000, Fig. 1 Chemical classification of volcanic rocks TAS (total alkalis vs. silica) diagram Covariation of other components Wohletz and Heiken, 1992, Fig. 1.2, adapted from Cox et al., 1979 Silica content Ultramafic IUGS divisions commonly followed for ultramafic to andesite No agreement on terms for silicic rocks <45 wt% SiO2 Basalt 45 – 52% Basaltic andesite 52 – 57% Andesite 57 – 63% Dacite 63 – 68% Rhyodacite (quartz latite) 68 – 72% Rhyolite 72 – 75% High-silica rhyolite 75 – 77.5% IUGS has only two terms for SiO2 > 63 wt% (dacite and rhyolite) Many people who work on non-alkalic silicic rocks use a subdivision similar to what is at left Silica content Ultramafic <45 wt% SiO2 Basalt 45 – 52% Basaltic andesite 52 – 57% Andesite 57 – 63% Dacite 63 – 68% Rhyodacite (quartz latite) 68 – 72% Rhyolite 72 – 75% High-silica rhyolite 75 – 77.5% Rogers and Hawkesworth, 2000, Fig. 1 Characterizing volcanic rocks Reminder about handout on minerals Begin Lecture 04 with further discussion of petrologic classification schemes (especially chemical) Now we will move on to physical properties Physical factors that influence volcanic processes Sigurdsson, 2000, Table 1 Physical properties of lava flows Kilburn, 2000, Table 2 Temperature T° Importance Influences magma viscosity (more later) Affects energy available for rise of eruption plume Units Kelvin (K) Celsius (°C) Measure directly with Optical pyrometer (mafic lavas only) Color when viewed with unaided eye Thermocouple Lockwood and Hazlett “Red” vs. “gray” volcanoes Optical pyrometer Essentially a telescope in which a wire filament is visible at same time as the glowing object (e.g., lava) Pass current through filament, causing it to glow Color of filament varies with strength of current Various corrections/calibrations required Have significant uncertainty Problems with smoke/haze Are not measuring T° of interior—only exterior crust Macdonald, 1972 Color viewed with unaided eye Use old principle Blacksmiths Operators of steel furnaces Temperatures related to color Valid when seen in dark (e.g., at night) Valid only if clear line of sight (not any intervening brownish fume clouds) Macdonald, 1972 Visual calibration at night Kilburn, 2000, Table 2 Thermocouple Method subject to the least error Pair of metallic wires of different composition welded together at both ends One end immersed in hot material Generates an electrical current in the circuit Strength of current depends on difference in T between hot and cold ends Cold end kept at 0° C with ice water bath Current measured with ammeter near cold end Can calculate T of hot end Practical limitations Lava too viscous to insert Thermocouple can be damaged by movement/flow Thermocouple Temperature measurements in an active lava flow at Mt. Etna, Italy Obtained with a thermocouple during an eruption in 1991 Stix and Gaonac'h, 2000, Fig. 11 Actual field measurements for eruption temperatures Tholeiitic basalt, Kilauea, HI 1050-1190°C Hawaiite, Mt. Etna, Italy 1050-1125°C Basaltic andesite, Parícutin, México 943-1057°C Dacite, Mount St. Helens, WA 850°C No data on rhyolites No eruptions measured or even viewed since Vulcan, Italy, in 1700’s Cas and Wright, 1987, Table 2.2 Temperature summary Composition Temperature (°C) Rhyolite-rhyodacite 700-900 Dacite 800-1100 Andesite 950-1170 Mafic (tholeiites) 1050-1250 Alkali basalts and nephelinites Ultramafic (komatiites) 900-1100 1400-1700 (est.) Williams and McBirney, 1979, Table 2-2; Cas and Wright, 1987, Table 2.3; Kilburn, 2000, Table 2 The high-T° end: Availability of any “superheat”? Aphyric rocks are unusual In other words, few, if any, lavas are hotter than the temperature at which they first begin to crystallize Exceptions Rare glassy basalts Some aphyric rhyolites (volatile-rich; heated by coeval hotter, underplating basalt?) “Superheat” generally not available, especially in silicic magmas (e.g., for melting rocks at or near the surface) Cannot cool without nucleating and growing crystals The low-T° end Erupted rocks are only partly crystalline Uncommon for volcanic rocks to have much >50% phenocrysts Why don’t we see cooler lavas with greater phenocryst contents? Limitations on the low-T° end Low-T end observed for a given composition probably corresponds to upper limit on viscosity for magmas of those compositions to remain mobile Migrate in crust Flow on surface Implies a “gap” in time between last extrusion from a magma chamber and its final crystallization as a pluton No geologic record for this interval Eruptive temperatures of prehistoric volcanic rocks No way to directly measure temperature Mineral geothermometers Return to in L04 Viscosity η Definition Resistance to flow, or Ratio of shear stress (σ) applied to a layer of thickness z to the rate at which it is permanently deformed in a direction x parallel to the stress Williams and McBirney, 1979, Fig. 2-1 Importance of viscosity Viscosity affects Fluidity of magmas and mobility of lavas Geometry and morphology of lavas and associated volcanoes Exsolution and nucleation of bubbles (vesiculation) Growth of bubbles Rise and escape of bubbles from magmas Fluid flow state: Laminar vs. turbulent Turbulent behavior of magmas (or pyroclastic and epiclastic aggregates) during flow is promoted by Increasing velocity Increasing irregularity of channel bottom and walls Decreasing viscosity more turbulent (i.e., more viscous less turbulent) We will return to this when we discuss lava flows, pyroclastic flows, pyroclastic surges, and lahars Classification of fluids on basis of rheology (viscosity, yield strength) Newtonian fluid (linear relationship) Zero yield strength (σ0=0) Linear relationship of shear stress to strain rate Good approximation for silicate melts (but not for multiphase suspensions or glasses) Bingham fluid (one of many non-Newtonian fluids) Finite yield strength (σ0>0) Linear relationship of shear stress to strain rate Good approximation for magmas Cas and Wright, 1987, Fig 2.3 Shear stress vs. strain rate Note: slopes = viscosity η Bingham fluids (magmas) If a stress less than the yield strength is applied (σ> σ0), resulting strain is Elastic (recoverable) If a stress greater than the yield strength is applied (σ> σ0), resulting strain has two components Elastic (recoverable) Viscous (non-recoverable) Viscosity η Units kg / m s = Pa s 1 poise = 1 g / cm / s = 0.1 Pa s (pascal second) Measure directly with penetrometers (data only for basalts) Estimate from velocities down channels (underestimates) Calculate from partial molar viscosities Pioneered by Bottinga and Weill and Shaw Viscosity η Melt viscosity issues Temperature [η ↓ with ↑ T] Dissolved volatile content, especially water content [η ↓ with ↑ H2O] Chemical composition, especially silica content [η ↑ with ↑ SiO2] Additional issues for magmas Rheological properties of magmatic suspensions (crystals, vapor bubbles) [η ↑ with ↑ volume fraction solids] Viscosity vs. temperature Log viscosity vs. temperature, as a function of composition (volatile-free) Rhyolites—more Si - O bonds to break Greater resistance to flow (higher viscosity) Basalts—fewer Si – O bonds to break Less resistance to flow (lower viscosity) Spera, 2000, Fig. 4 Viscosity vs. dissolved water content Log viscosity vs. dissolved water content, as a function of composition Spera, 2000, Fig. 5 Viscosity comparison e.g., Hawaiian tholeiite 1200°C 1130°C By comparison, H2O 25°C η = 500 poise = 50 Pa s η = 8000 poise = 800 Pa s η = 0.01 poise = 0.001 Pa s If basalts are much more viscous than water, why, then, do basalts flow fairly rapidly? Viscosity: Network formers and Network modifiers Network formers contribute to η ↑ Network modifiers contribute to η ↓ Si, Al Network formers (strong bonds with O) (η ↑) Fe, Mg, Ti, others Network modifiers (η ↓) Alkalis: Na, K, Rb, Cs Network formers in peraluminous and metaluminous melts (η ↑) Network modifiers in peralkaline rocks (η ↓) Volatiles: H2O, F, Cl Network modifiers (η ↓) Why do basalts flow fairly rapidly? Aided by gravity Flow down slopes of a shield volcano High density Have a density considerably greater than water Viscosity vs. dissolved water content Log viscosity vs. dissolved water content for rhyolitic / granitic melts, as a function of temperature Wallace and Anderson, 2000, Fig. 14 Viscosity vs. volume fraction solids Log viscosity vs. volume fraction solids Spera, 2000, Fig. 6 Viscosity changes during flow Typically increases by 2 to 10X from vent to toe of flow Primarily because of loss of volatiles Minor effect of cooling Density ρ Definition: mass per unit volume Units kg / m3 g / cm3 Melt density is a function of Temperature [ρ ↓ with ↑ T] Pressure [ρ ↑ with ↑ P] Dissolved water content [ρ ↓ with ↑ H2O] Density decreases (volume increases) on melting Changes in density ρ Temperature dependence of density Coefficient of thermal expansion Similar for most compositions: ~2 – 3 X 105 deg-1 Pressure dependence of density Compressibility Increases sharply in the melting range Density vs. temperature Density of melt vs. temperature, as a function of composition Spera, 2000, Fig. 1 Density vs. pressure Density of model basaltic melt vs. pressure, for temperatures of 1800 and 2800°C Spera, 2000, Fig. 3 Density vs. dissolved water content Density of melt vs. dissolved water content, as a function of composition Spera, 2000, Fig. 2 Density summary (at liquidus temperature and anhydrous, except as noted) Composition Granite / rhyolite Granite / rhyolite (2 wt% H2O) Granodiorite / dacite Gabbro / basalt Komatiite Liquidus Density Density T° (°C) (kg/m3) (g/cm3) 900 2349 2.35 900 2262 2.26 1100 2344 2.34 1200 1500 2591 2748 2.59 2.75 Spera, 2000, Table 3 Importance of density Important control on rise of magmas through crust Strong control on fluid dynamics of magmas Petrologic implications for mixing of magmas Transport of magmatic heat Convection Heat transported by bulk flow Conduction (phonon conduction) Phonon = quantized thermal waves Heat transported by atomic vibration of lattice Radiation Electromagnetic phenomenon involving photon transfer Thermal conductivity k If k = thermal conductivity κ = thermal diffusivity ρ = density Cp = specific heat, Then the thermal conductivity k = ρ Cp κ Units J / (m K s) = W / (m K) Most melts, rocks, and minerals are characterized by low thermal diffusivity and thermal conductivity Specific enthalpy of fusion Δhf Definition Heat per unit mass needed at constant pressure to transform a crystal or crystalline assemblage to the liquid state Units kJ / kg Very high for magmas, with wide variation 100 – 300 kJ / kg for crustal phases ~1000 kJ / kg for refractory phases that are components of mafic and ultramafic melts Enthalpy of fusion--Implications Anatexis of crust by heat exchange between mafic magma and crust is thermally efficient Heat required to completely melt Earth’s mantle, 3 x 1030 J Is <10% of the kinetic energy delivered to Earth by impact of a Mars-sized body (15% of mass of Earth) with an impact velocity equal to Earth’s escape velocity of 11.2 km/s Molar isobaric heat capacity Cp Definition Heat needed at constant pressure to raise temperature of one mole by one Kelvin Units J / kg K Low for magmas (< half that of water) Silicic anhydrous melts 1300 – 1400 J / kg K Mafic – ultramafic anhydrous melts 1600 – 1700 J / kg K Implies mafic and ultramafic magmas are better transporters of magmatic heat Crystallization rates Rate decreases as viscosity increases Rate ↓ with ↑ η Consequences Rhyolites (high η) crystallize slowly glassy groundmass Basalts (low η) crystallize rapidly fine crystalline groundmass Recrystallization of glass Rhyolitic glass silica mineral + alkali feldspar (and/or clay minerals and zeolites in alkaline lakes) Hydrate and crack Nucleate crystals along cracks Summary The time, length, area, volume, and energy scales of volcanism and volcanic rocks Each vary by many orders of magnitude, but Characteristic features vary within fairly narrow ranges Mineralogy is a function of chemical composition Silica content and alkalinity are key compositional variables The most important physical properties are Temperature T°, Viscosity η, Density ρ, Thermal conductivity k, and Crystallization rates Impacts on viscosity η ↓ with ↑ T; η ↓ with ↑ H2O and most other volatiles; η ↑ with ↑ SiO2; η ↑ with ↑ volume fraction solids (e.g., phenocrysts) The properties are not independent of one another Many can be linked to chemical composition of the magma Many observations can be explained in terms of viscosity (e.g., shapes of volcanoes, eruptive style) Next time: Volatiles