Elucidating Reaction Pathways for Thermoelectric Materials Fabricated by
advertisement

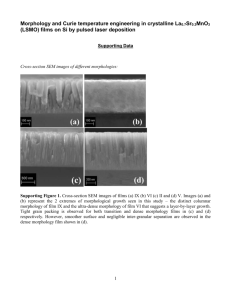
Presented ACS 2015 Spring National Meeting, Denver, CO Elucidating Reaction Pathways for Thermoelectric Materials Fabricated by Bottom-Up Solution-Phase Solid-State Synthesis Cameron Holder, Evan Rugen, Daniel Stevens, and Mary E. Anderson* Introduction Weigh out stoichiometric amounts of TeO2, Pb(C2H3O2)2, and Bi(NO3)3 Thermoelectrics convert heat to electricity or vice versa Add salts to 10 mL of solvent Tetraethylene glycol (TEG) Reduce cost and energy consumption Sparge solution with nitrogen (N2) for ~15 minutes Increase efficiency Combine NaBH4 with 5 mL of TEG and pipet into flask 20 30 EDS Spectra for PbTe 40 2θ 50 60 Identifies crystal structure and indicates composition Store powder under vacuum to dry in order to characterize using SEM/EDS and XRD Synthetic Method: Modified Polyol Process Te+4 XRD Pattern of TeO2 Identifies elemental composition of sample Wash by centrifugation three times with ethanol Methods X-ray Diffractometry (XRD) Energy Dispersive X-ray Spectroscopy (EDS) Pipet solution of product into centrifuge tubes Seat Warmers/Coolers, Refrigeration, Deep Space Probes Pb+2 Scanning Electron Microscopy (SEM) Run reaction under N2, for specified temperature and amount of time Promising applications: Te+4 Characterization Techniques Experimental Details PbTe and Bi2Te3 nanoparticles are classified as thermoelectric materials Pb+2 Department of Chemistry, Hope College, Holland MI 49423 Experimental Goals Intermetallic Nanoparticles Summary TEG and NaBH4 Te+4 PbTe Heat Pb+2 Determine the growth mechanism of PbTe and Bi2Te3 nanoparticles as a function of time and temperature Acknowledgments • The PbTe mechanism forms an oxide intermediate, while the Bi2Te3 mechanism proceeds through a reduction-diffusion process. • The growth mechanism for both materials remains unchanged when the reaction was run for a longer period of time (1 hour). However, growth stages were observed at lower temperatures. Lab members: Monica Ohnsorg, Lauren Gentry, Brandon Bowser Hope College Chemistry Department NSF HHMI Results Pb0 PbTe TeO2 PbTeO3 24°C Bi2Te3 TeO2 PbTe, PbTeO3 PbTeO3, PbTe PbTe 24°C 270°C 100°C TeO2 280°C 220°C 200°C 150°C 185°C Bi2Te3 150°C 100°C 250°C 250°C Te 220°C 220°C 185°C 200°C TeO2 200°C 20 200°C 100°C 220°C 24°C 280°C PbTe Bi2Te3 PbTeO3 Bi Pb 150°C TeO2 40 50 2θ 60 70 20 80 Above: PbTe XRD pattern where reactions were held at the indicated temperature for 1 minute. 200°C • Upon the application of heat, the Pb begins to diffuse into the TeO2 crystal, forming the bulbous PbTeO3 intermediate. 35 40 2θ 45 50 55 60 • Bi2Te3 mechanism begins much like what was observed for PbTe mechanism with elemental Bi and TeO2 after NaBH4 addition without heating. 220°C • While PbTe goes through an oxide intermediate, the Bi2Te3 been shown to proceed through a reduction-diffusion process. • Distinct morphology changes are observed for PbTeO3 before oxygen is expelled from the crystal to from the final product, PbTe. 270°C 30 Above: Bi2Te3 XRD pattern where reactions were held at the indicated temperature for 1 minute. • At room temperature and after the NaBH4 addition, the Pb precursor is reduced and the TeO2 is unchanged. 250°C 25 • There comes a point where rate of reduction exceeds rate of the Bi-Te reaction and Te nanorods are observed (220°C). Pb(C2H3O2)2 30 All scale bars are 10 µm. All scale bars are 10 µm. 45 50 55 60 Std. Open Cl2 NO3 PbTe PbTeO3 5 μm 5 μm Pb TeO2 Te 20 5 μm 5 μm PbCl2 250°C Left: Morphologies observed at each stage of the Bi2Te3 1 minute growth mechanism. From the top to bottom, the following are the EDS percentages for Bi and Te, respectively, for each growth stage: 24°C: 20%, 80%; 100°C: 45%, 55%; 150°C: 42%, 58%; 200°C: 26%, 74%; 220°C: 16%, 84%; 250°C: 36%, 64%. 40 2θ Closed • The formation of Bi2Te3 nanoplatelets is complete at 250°C. Left: Morphologies observed at each stage of the PbTe 1 minute growth mechanism. The following are the EDS percentages for each growth stage, from top to bottom, referring to Pb and Te, respectively: 24°C: 62%, 38%; 175°C: 12%, 88%; 200°C: 36%, 64%; 220°C: 43%, 57%; 250°C: 49%, 51%; 270°C: 51%, 49%. 35 PbTeO3 Pb(NO3)2 Te 220°C All scale bars are 10 µm. Te TeO2 20 30 • Reduction of TeO2 investigated to determine its role in formation of Bi2Te3. • Distinct morphological transitions are observed in SEM images (left) from 200°C to 220°C, which are also seen for Bi2Te3. • Representative XRD patterns (above) show progression of TeO2 reduction as a function of temperature. 100°C 24°C 200°C 25 150°C 175°C 175°C TeO2 Te Te Bi0 5 μm All scale bars are 10 µm. 5 μm 30 40 50 2θ 60 70 80 • Formation of PbTeO3 was dependent on conjugate anion of Pb starting salt containing oxygen atoms as observed in both the SEM images (left) and the XRD patterns (above). • It was determined that formation of PbTeO3 was independent of atmospheric conditions. Reactions were run under a static (closed) and dynamic (std) N2 environment in addition to being run open to the atmosphere (open). Presented ACS 2015 Spring National Meeting, Denver, CO [selected for Sci-Mix, students won COLL best student poster award] Foundational Layer Formation of Metal-Organic Coordinated Thin Films Monica L. Ohnsorg, Brandon H. Bowser, Lauren K. Gentry, Christopher K. Beaudoin and Mary E. Anderson* Department of Chemistry, Hope College, Holland MI 49423 Introduction Layer-by-Layer Deposition of Metal-Organic Multilayers vs. Frameworks SPM Images Layer-by-Layer Deposition Methods Cu-TMA 25˚C 1 O This research explores layer-by-layer (LBL) assembly for two types of metal-organic coordinated thin films, multilayers (ML) and frameworks (MOF). Controlled step-wise assembly defines the resulting film structure, presenting an opportunity to design these materials for specific applications, such as sensing and gas storage. Towards this realization, both films are fabricated by alternating, sequential solution-phase deposition. Both systems were synthesized beginning with a 16-mecaptohexadecanoic acid (MHDA) self-assembled monolayer on gold. ML were composed of α,ω-mercaptoalkanoic acids and Cu (II) ions forming a conformal film. MOF were composed of 1,3,5-benzenetricarboxylic acid and Cu (II) ions (HKUST-1) yielding a porous, crystalline framework. Both films were characterized using ellipsometry to measure film thickness and scanning probe microscopy (SPM) to map topographical morphology of film growth LBL. Using image analysis software, quantitative data regarding the growth of these thin films based on the images was procured. Ellipsometry suggests both ML and MOF form continuous, conformal layers that are each about 2 nm thick, respectively. However, SPM images elucidate two distinct systems, one that forms a semi-continuous film with distinct “islanding” (ML) and one that forms a rough surface of nucleating crystallites (MOF). The effects of deposition conditions, such as temperature and solution concentration, have been investigated in order to tailor film morphology for specific applications. Preliminary findings will be presented for utilizing surface IR for gas absorption within the MOFs. Future work includes observing continued MOF growth to observe at what point it becomes continuous and to investigate how the film forms beyond the threshold of complete surface coverage. Further studies will investigate other metal-organic coordinated thin film systems to understand the chemical and physical processes by which different film morphologies arise. 2 4 3 5 10 16 20 25 30 Cu2+ (a) ML HS 25°C Metal-organic multilayers form as a conformal continuous film covering the underlying substrate completely. After 1 deposition cycle, a foundational and conformal layer covers the grain structure of the underlying Au substrate observed. Then islands begin to form and coalesce throughout the next few deposition cycles. At ~5 cycles, the film takes on a unique morphology but surface roughness is essentially the same as the underlying substrate. Graph (a) shows linear growth of 1.8 nm per layer and fairly consistent, low roughness measurements are shown in Graph (c). 1 Cu2+ 3 2 4 5 8 7 6 9 200 nm 10 50 nm (b) 25°C HKUST-1 SurMOF - small crystallites are present on the surface after 1 cycle of deposition; and the crystallites double in number from 1 to 2 cycles. The crystallites continue to nucleate, grow in size increasing surface coverage (Graph (b)), and coalesce with one another (9 and 10 cycles). Graph (a) shows growth comparable to that of the ML. Roughness increases with deposition cycle (Graph (c)). 1 Cu2+ 4 3 2 5 6 8 7 9 2.00 µm 10 50 nm (c) 50°C OH OH O OH O OH O O 2) Copper (II) Perchlorate Hexahydrate (1 mM, 15 min) OH O 1) 16 - Mercaptohexadecanoic Acid (1 mM, 1 hr) Increasing deposition temperature from 25˚C to 50˚C resulted in nucleation of smaller and more numerous crystallites that appear to almost fully cover the surface at 10 cycles of deposition. Depositions increase roughness up to cycle 6 (Graph (c)). The thickness of the film is comparable to the 25˚C data up to 5 cycles of deposition, after which the film increases in thickness at a faster rate. Applications: Lithography Au OH O S S S S Au Anderson et al., Adv Mater, 2006, 18, 1020. OH OH O S 1 Zn2+ S OH O S S OH O S S S S O S OH S OH O S OH O OH O S OH O OH O S OH O OH O S O OH O S OH O S 2 3 6 Preliminary data for substituting the metal ion in the SurMOF system with Zn2+ resulted in the formation of platelet-like crystallites on the surface, resulting in roughness measurements much lower than the Cu SurMOF systems (Graph (c)). The thickness of the film, shown in Graph (a), was approximately half that of the 25˚C deposition of the Cu SurMOF film. Tracking Gas Adsorption IR Spectra 0.010 HO O OH O O 0.04 0.02 0.020 0.010 H2O + -OH of COOH 0.030 Cu-HKUST Cu-HKUST + NH3 0.006 0.004 0.002 3500 3100 1700 1300 Wavenumbers (cm-1) 3500 3100 1700 1300 Wavenumbers (cm-1) http://www.moftechnologies.com/MOFs.html http://http://www.sigmaaldrich.com S S S S S S S 1700 S Scanning Probe Microscopy Laser Electronics Tip Ellipsometry Particle Analysis 1500 1450 1400 1350 3600 3400 3200 3000 2800 1800 1600 1400 1200 1000 800 Subtracted Background Original Particle Count Particle Size Percent Coverage Particle Outline Particle Threshold Absorbance spectra for deposited layers elucidates chemical composition of the films and monitors layer growth by measuring intensity changes in the observed functional group peaks. 200nm Thickness: 6.75 nm Rq: 14.4 nm Cu-TMA 25˚C Deposition on Template Stripped Au 3 50 nm 2µm 20 nm Comparison of the Cu- and Zn-HKUST systems. Spectra above represent samples fabricated by 5 deposition cycles. Key peaks are identified, supporting the chemical composition of the film. Thickness: 8.02 nm Rq: 20.5 nm 2 1 Wavenumbers (cm-1) Investigate other SURMOF systems Cu-Jungle Gym System MOF-5, Jungle Gym Understand fundamentals of framework formation Explore crystal face expression and formation Develop growth mechanism Tailor film structure via deposition variables Test variables such as time, temperature, solvent, and concentration Introduce functionality into films Dope MOFs for post-synthetic functionalization Induce conductive or magnetic properties Cu2+ Preliminary integration into smart interfaces ITO-PET Track absorption of gases into framework Funding: 20 nm 50nm 50nm 2.00µm 2.00µm Zn-HKUST Post-NH3 Cu-HKUST Post-NH3 Future Investigations Grazing Angle FT-IR Plane polarized light of interacts with the surface. The ratio of change from plane to elliptically polarized light estimates film thickness and optical properties. 1550 Wavenumbers (cm-1) Computer In SPM images, bright areas indicate taller features while dark areas represent lower regions. 1600 Film growth can be qualitatively and quantitatively investigated throughout the deposition of the Cu-HKUST SurMOF system. Interactions between the scanning probe and surface renders a topographic, qualitative image of film morphology on the nanoscale. Cantilever Sample 1650 Wöll et al., Angew. Chem. Int. Ed. 2009, 48, 5038. Characterization Methods Detector 4 4 S 10 Thickness: 2.85 nm Rq: 7.76 nm 0.010 0.000 0.000 S 5 500 nm Cu-TMA 25˚C NOTE: Regions with fewer crystallites were selected in order to investigate background substrate morphology. 0.020 N—H Absorbance 0.030 0.008 Zn-HKUST Zn-HKUST + NH3 0.00 Biomedical – Drug Storage, Delivery 2 2.00 µm The underlying, rippled morphology of the substrate remains consistent through 10 cycles of deposition, indicating minimal disruption of the preliminary SAM. N—H OH HO OH O O Cu-HKUST Zn-HKUST 1,3,5- sub benzene HO O O 0.06 0.040 Absorbance O OH OH HO OH O O O TMA match HO O O HO O OH O OH O HO O O OH HO OH O OH OH O OH O Hydrocarbon Separations HO OH HO HO O OH HO OH O O HO O O benzene HO O Absorbance O OH O COO- assym 0.08 Layer 1 Layer 2 Layer 3 Layer 4 Layer 5 Layer 6 Layer 7 Layer 8 Layer 9 Layer 10 COO- sym 3) Organic Ligand: Benzene-1,3,5-tricarboxylic acid (0.1 mM, 1 hr) Characterization and Comparison COO- sym Watching the SURMOF Grow COO- assym benzene TMA match 2) Metal Ion: Copper (II) Acetate or Zinc (II) Acetate (1mM, 30 min) O 1 Au 1) 16 - Mercaptohexadecanoic Acid (1 mM, 1 hr) O 10 25°C Metal-Organic Framework (MOF) Applications: Gas Storage 9 8 7 2.00 µm 50 nm Evans et al., JACS, 1991, 113, 5866 Carbon Capture and Sequestration 5 4 -CH2 (SAM) Electronic and Nanofluidic Devices Zn-TMA 25˚C 20 nm Metal-Organic Multilayer (ML) Chemical Functionality Cu-TMA 50˚C ML OH ABSTRACT 200nm 200nm Thermally Deposited Au Template Stripped Au with MOF nano-crystallites Investigate effects of grain boundaries on crystallite nucleation. Results followed average trends and crystallites nucleate independent of etch pit boundaries and Au grains. Conclusions 4 50nm ML form conformal film with unique and stable morphology emerging after 5 deposition cycles, having surface roughness equal to underlying substrate. LBL deposition at 25˚C for Cu-TMA SurMOF resulted in a crystallite-rich surface different from the ML system. Increased deposition temperature for SurMOF increased surface coverage of particles which were more numerous and smaller in size - resulting in greater surface coverage, increased thickness, and lower roughness. 2.00µm Changing the metal ion results in new crystallite structure and decreased roughness. 10 50nm Nucleation of crystallites from Cu-TMA SurMOF deposited at 25˚C is independent of grain boundaries on Au substrate. IR showed layer growth for Cu-TMA SurMOF as functional group intensities increased. NH3 absorption was observed using IR. SPM showed SurMOF morphology had been disrupted by the gas. 2.00µm ACS – PRF Arnold and Mabel Beckman Foundation NSF-MRI Grant No. CHE-1126462 Hope College Chemistry Department Group Members: Allie Benson Evan Rugen Dan Stevens Meagan Elinski Cameron Holder Presented ACS 2013 Spring National Meeting, New Orleans, LA [selected for Sci-Mix] Qualitative and Quantitative Analysis of Metal-Organic Coordinated Multilayers Meagan B. Elinski, Alexandra S. Benson, Mary E. Anderson* Department of Chemistry, Hope College, Holland MI 49423 Introduction Results Particle Induced X-Ray Emission (PIXE) 10000 Bottom-up Assembly of Multilayer Films Cu+X Cu+X Cu+X Cu+X Cu Au 0.004 Au Cu+X Cu+X Cr Cu 400 200 300 Channel/Energy 100 0.002 0.000 Energy 0 10 20 30 Layer 600 500 400 300 200 100 0 5 0L 2 Cu: 1 molecule 10 15 20 # of Layers 25 Fitting Cu peak suggests 2-to-1 Cu-to-molecule ratio 30 Average density per layer: 2.21 x1014 molecules/cm2 5L 1000 10L 15L 100 10 1 Cu: 1 molecule Film Thickness 0 10000 Linear increase in Cu concentration 15-30 layers Cu+X 10 1 1E15 atoms/cm2 Counts Au Au Si 100 0.006 Counts How does the morphology and roughness change with increasing number of multilayers? Are there trends observed to glean insight into film formation? Count s Cr Au 1000 30 Layer Si What is the average areal density of individual layers? What is the topography of the multilayer films? Rutherford Backscattering Spectrometry (RBS) Cu/Au What is the composition and structure within the films? What is the binding ratio between metal ions and molecules? 20L 455 460 465 Channel/Energy 470 Fitting shift in front Au edge shows increase in film thickness Standard monolayer density: 4.5x1014 molecules/cm2 Cu+X Atomic Force Microscopy (AFM) 3: 2nd layer Evans, et. al. J. Am. Chem. Soc. 1991, 113, 5866-5868. Atomic Force Microscopy Peakforce tapping mode to map topography Herein used to study surface morphology as a function of layer deposition and annealing temperature Particle Induced X-ray Emission (PIXE) Ion beam: protons (H+), 2.9 MeV particle accelerator Detects emitted X-rays Determines elemental composition in ppm Rutherford Backscattering Spectroscopy (RBS) Ion beam: alpha particles (He1+), 2.9 MeV particle accelerator Detects energy loss of backscattered particles Determines elemental composition: based on nuclear collisions Calculates thickness (atoms/cm2): based on electronic collisions incident particles multilayers Au Cr backscattered particles SiO2 Roughness Analysis: Nonlinear curve fit (Ws) Roughness Analysis: Image analysis software (Rq) 1L 0L Rq: 1.52 nm Ws: 1.59 nm 2L Rq: 1.60 nm Ws: 1.69 nm 3 Layer 3L Rq: 1.93 nm Ws: 1.95 nm 10 L 4L 5L Rq: 2.21 nm Ws: 2.29 nm Rq: 2.00 nm Ws: 2.04 nm 20 L 25 L 16 L Rq: 1.54 nm Ws: 1.59 nm 30 L Each image is 500x500 nm Chris Beaudoin, Cameron Holder, Evan Rugen, Kyle Alexander Dr. Graham Peaslee, Dr. Paul DeYoung, Dr. Jennifer Hampton Hope College Chemistry Department National Science Foundation Howard Hughes Medical Institute NSF-MRI Grant No. CHE-1126462 3L film: greater height deviation within smaller areas (boxes); short range disorder 30L film: same overall roughness as 3L; exhibited over larger areas Length of Box (µm) Rq: 1.50 nm Ws: 1.62 nm Rq: 1.67 nm Ws: 1.74 nm Rq: 1.53 nm Ws: 1.62 nm Rq: 1.99 nm Ws: 2.20 nm Rq: 1.67 nm Ws: 1.79 nm Heating in situ (1 x 1 μm scans of 3 Layer Multilayer Sample) Images are labeled with the sample stage temperature and measured surface roughness (Rq) Controlled heating from room temperature to 200°C and subsequent cooling to ambient temperature 25°C 75°C 100°C 150°C 200°C 100°C 25°C side view Acknowledgments 30 Layer 3 Layer Growth 30 Layer Growth Roughness (nm) 2+ metal ions Cu 2: 1: MHDA: (16-mercaptohexadecanoic acid) Two methods of roughness analysis were compared Numbers represent deviation in height from a mean value over a specified region Rq: 1.65 nm Rq: 1.47 nm Multilayer film SPM images before and after ex situ annealing to 100 °C. Rq: 1.21 nm Rq: 0.968 nm Pre3L Rq: 3.04 nm Rq: 2.53 nm Post-annealing Pre- 3L 30L Rq: 2.36 nm Post-annealing 30L Each scan is 500 x 500 nm Rq: 1.97 nm Rq: 1.62nm Rq: 1.78 nm Rq: 1.80nm Conclusions and Future Work What is the composition and structure within the films? Cu concentration increases linearly with film growth Binding ratio of Cu to MHDA is approximately 2:1 Average density per layer similar to SAM What is the topographical structure of the films? Foundational layers conformal with underlying substrate Film takes on unique morphology upon reaching five layers Surface roughness increases with initial layers, but decreases to that of underlying substrate Next steps: Examine island sizes as multilayers form Study temperature range in which transformation occurs (75ºC-125ºC) using a tip more appropriate for imaging organic films Investigate other metal-organic coordinated systems Multilayers: e.g. alkylphosphonic acids and Zr4+ or Zn2+ Metal-organic frameworks: e.g. trimesic acid and Cu2+