1910 Nobel Prize RT pV a

pV
RT
1910 Nobel Prize van der Waals
( p
a
V
2
)( V
b )
RT
On the continuity of the gaseous and liquid state (1872)
… if a gas in the extremely dilute state, where the volume is so large that the molecules can be regarded as points, consists of small moving particles, this is obviously still so when the volume is reduced; indeed, such must still be the case down to the maximum compression and also in liquids, which can only be regarded as compressed gases at low temperature. Thus I conceived the idea that there is no essential difference between the gaseous and the liquid state of matter - that the factors which, apart from the motion of the molecules, act to determine the pressure must be regarded as quantitatively different when the density changes and perhaps also when the temperature changes, but that they must be the very factors which exercise their influence throughout. And so the idea of continuity occurred to me.
December 12, 1910 Nobel Lecture
Although I have not as yet seriously gone into that question I do think that in the amorphous state the close proximity of the molecules impedes their mutual displacement.
The crystalline state definitely behaves in a slightly different way. Actually I should still be silent on this question. …. As you are aware the two factors which I specified as reasons why a non-dilute aggregate of moving particles fails to comply with Boyle’s law are firstly the attraction between the particles, secondly their proper volume.
Thermodynamic Stability
p
V
T
T
0
1
V
V
p
T
0
Kinetic Theory - Basic assumptions
1. A macroscopic volume contains a large number of molecules.
2. The separation of molecules is large compared with molecular dimensions and with the range of intermolecular forces.
d d
0.0002 to
0.05 eV d
( p
V a
2
)( V
b )
RT
Thermodynamic instability in regions where:
p
V
T
T
0
1
V
V p
T
0
Where are we going?
Critical Point
Law of Corresponding States
(Liquid-gas coexistence)
Where are we going?
Critical Point
PVT surface for a real substance
Regions where Van der Waals’ PVT surface is multi-valued is where one finds co-existence of phases.
Equation of State of a Real Gas
Van der Waals’ equation in intensive form:
100
P
v a
2
v b
90
80
70
60
50
CP
40
30
20
10
0
12 o
10 o
9 o
8 o
6 o
4 o
0.1
0.2
0.3
0.4
0.5
0.6
Specific volume (arb. units)
11 o
9.5
o
8.5
o
7 o
5 o
3 o
RT
van der Waals Coefficients
Gas
Helium a (m 3 Pa) b (m 3 mol)
3.46 x 10 -3 23.71 x 10 -6
Neon
Hydrogen
2.12 x 10 -2 17.10 x 10 -6
2.45 x 10 -2 26.61 x 10 -6
Carbon dioxide 3.96 x 10 -1 42.69 x 10 -6
Water vapor 5.47 x 10 -1 30.52 x 10 -6
Data from Fishbane, et al.
Normalized volume and Gibbs Free energy as a function of normalized pressure
Isotherm at
T = 0.9T
c
Fig. 26.3 of Blundell
G p
C p
T
P v
T
P v
T
P v
T
P v
T
P v
T
P v
T
P v
p
F
F
1
F
2 p o
V
1
+
-
T < Tc
p / V
T
0
2
F /
V
2
T
0
+
V
2
F /
V
2
T
0 for stability
V
2
Common tangent line
V
Expansivity and Compressibility
Two important measurable quantities:
Expansivity:
1 v
v
T
P
Compressibility:
1 v
v
P
T
Water: CP at 374
C and 218 atm
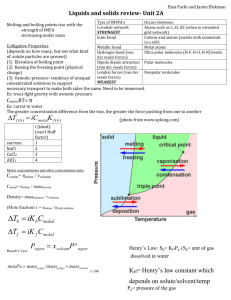
Phase Diagram of CO
2
Critical Point
Triple Point
CO
2
G/p c
V c
G(p,T) of vdW gas
T < T c
T = T c
T > T c
1 p/p c
G(p,T) of vdW gas
p
T
Equation of State of a Real Gas
Van der Waals’ equation in intensive form:
50
P
a v
2
v b
RT
40
30
20
10
0.1
0.2
0.3
0.4
0.5
Specific volume (arb. units)