The van der Waals gas
advertisement

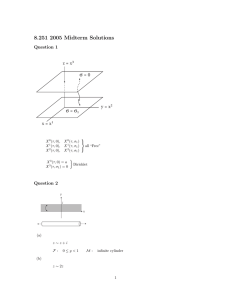
The van der Waals gas Assuming that from the notes you can write the partition function as Z= 1 −3N N (N − 1) N −2 λ V VN + N! 2 Z d3 x1 d3 x2 f12 where f12 = f (|x1 − x2 |) depends only on the separation between x 1 and x2 . This suggests a change of variable to center of mass coordinates. Write x = (x1 − x2 )/2 and r = x1 − x2 . Then, Z d3 x1 d3 x2 f12 −→ V Z d3 r e−βV (r) − 1 where e−βV (r) − 1 = f (r). Writing, 1 −3N λ N! Z = = ( V N 1 −3N N λ V N! ( N 2 V N −1 + 2 Z 3 d rf (|r|) N2 1+ κ(β, a, L) 2V ) ) where κ(β, a, L) = 4π r 2 drf (|r|) = 4π r 2 dr(e−βV (|r|) − 1). This integral can be evaluated to give κ(β, a, L) = −A + Bβ with A = 4πa 3 /3, B = 4π 3 3 3 ((a + L) − a )V0 . Then writing, R F R = −1 ln Z β = N2 −1 ln Z 0 1 + κ(β) β 2V ( !) where Z 0 = λ−3N V N /N ! is the partition sum for the perfect gas. Assuming that V0 and a are small so that N 2 κ/2V ≤ 1. Then, F ∼ 1 N 2κ 1 1 N2 −1 ln Z 0 − · = − ln Z 0 − (−A + Bβ). β β 2V β β 2V The equation of state for a gas with interactions of this type is δF P =− δV = T N kT N2 − (−A + Bβ) V 2βV 2 1 which, substituting the expression for A and B, gives, N kT + P = V or N 2B P+ 2V 2 N 2A 2V 2 ! ! · kT − NA V − 2 N 2B 2V 2 = N kT. This is the van der Waals equation of state. It describes a realistic gas a 2 low density with a shallow attractive potential ( N2Vκ ≤ 1). 2