Thermodynamics Universe Surroundings System
advertisement
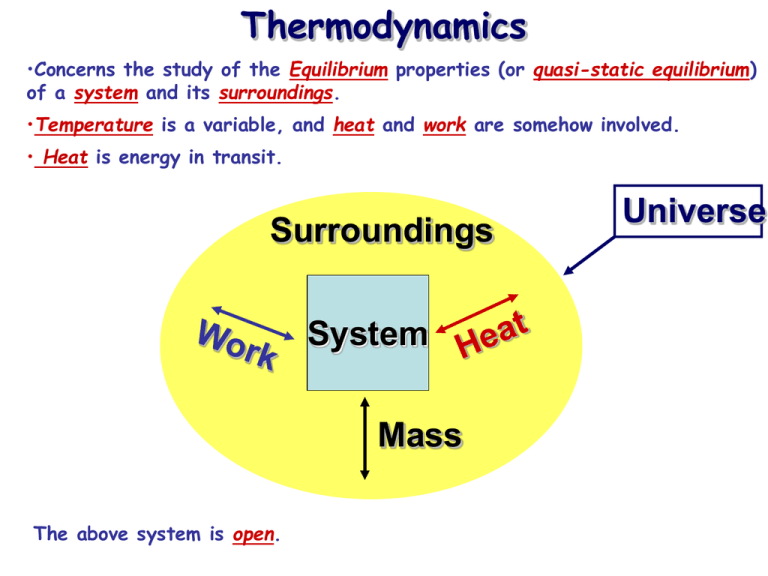
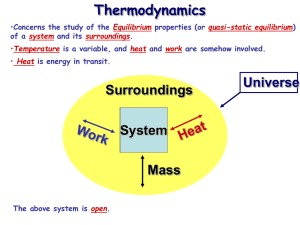
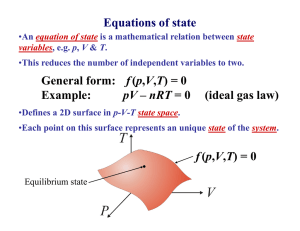
Thermodynamics •Concerns the study of the Equilibrium properties (or quasi-static equilibrium) of a system and its surroundings. •Temperature is a variable, and heat and work are somehow involved. • Heat is energy in transit. Surroundings System Mass The above system is open. Universe Thermodynamics •Concerns the study of the Equilibrium properties (or quasi-static equilibrium) of a system and its surroundings. •Temperature is a variable, and heat and work are somehow involved. • Heat is energy in transit. Surroundings System The above system is closed. Universe Thermodynamics •Concerns the study of the Equilibrium properties (or quasi-static equilibrium) of a system and its surroundings. •Temperature is a variable, and heat and work are somehow involved. • Heat is energy in transit. Surroundings System Mass The above system is undergoing an adiabatic change. Universe Thermodynamics •Concerns the study of the Equilibrium properties (or quasi-static equilibrium) of a system and its surroundings. •Temperature is a variable, and heat and work are somehow involved. • Heat is energy in transit. Surroundings System The above system is isolated. Universe Thermodynamics •Concerns the study of the Equilibrium properties (or quasi-static equilibrium) of a system and its surroundings. •Temperature is a variable, and heat and work are somehow involved. • Heat is energy in transit. Surroundings Universe System System Wall The above system is isolated. Thermodynamics •Concerns the study of the Equilibrium properties (or quasi-static equilibrium) of a system and its surroundings. •Temperature is a variable, and heat and work are somehow involved. • Heat is energy in transit. Surroundings Universe System System Wall The above is an example of a diathermal wall. Equations of state •An equation of state is a mathematical relation between state variables, e.g. p, V & T. •This reduces the number of independent variables to two. General form: f (p,V,T) = 0 Example: pV – nmRT = 0 (ideal gas law) •Defines a 2D surface in p-V-T state space. •Each point on this surface represents an unique state of the system. f (p,V,T) = 0 Equilibrium state: macroscopic variables do not change in time! Joule’s apparatus for measuring the mechanical equivalent of heat 1 cal = 4.184 J will raise temperature of water by 1 C (14.5 C to 15.5 C) Specific heats of ideal gases • Now that we have an expression for the internal energy of an ideal gas, we can calculate the specific heats: du f u cv R T v dT 2 cP cv f 2 f f cP cv R 1 R 2 f 2 1 2 f f 3 .