CHEM 102 General Chemistry - II Lecture Course Instructor
advertisement

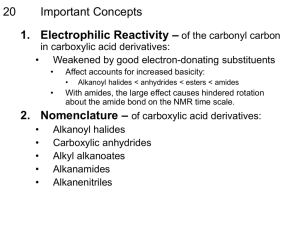
CHEM 102 General Chemistry - II Spring 2016 Section 01 Lecture Days: MWF Time: 11:00 – 11:50 am Place: Currens 315 Course Instructor Dr. Shaozhong Zhang Office: 430-B Currens Hall Phone: 309-298-1685 Email: s-zhang@wiu.edu Office hours: MWF (9:30 – 10:40 AM), or by Appointment Course Prerequisite Chem 101 or equivalent (C grade or better, strictly enforced) Students without the prerequisite will be dropped from the course Course Description This is a continuation of CHEM 101 course. CHEM 102 course deals with the application of the general principles of organic chemistry to biological, environmental, and applied sciences. It includes the study of nomenclature, preparation, reactions of the functional groups of aliphatic and aromatic compounds, elementary biochemistry. Course Objective • To know and understand the bonding and structure of organic compounds • To appreciate the dependence of molecular properties on that bonding and structure • To know the reaction of organic • To develop practical laboratory experience in modern organic chemistry Outside work requirements for the course Students are expected to review, study and learn all material discussed in lecture, as well as read assigned chapters in the textbook, and to work assigned practice problems/questions/terms listed in class or on Western Online. Generally a minimum of 2 to 3 hours of outside study time is required for each hour of class time for this course. Text and Related Material Introduction to General, Organic, and Biochemistry, 11th edition By Bettelheim, Brown, Campbell, and Farrell. Thomson Brooks/Cole. ISBN # 978-133-10508-4 1 Assessment of Students (Tentative) Quizzes (10) Exams (4) Laboratory Final Exam Total 200 Points 400 Points 250 Points 150 Points 1000 Points Course Grading Scale: Total Points Letter Grade 930-1000 A 740-769 C 900-929 A- 700-739 C- 870-899 B+ 650-699 D+ 840-869 B 600-649 D 800-839 B- 500-599 D- 770-799 C+ 0 - 499 F LECTURE TESTS AND QUIZZES Four lecture exams (each worth 100 points), ten quizzes (each worth 20 points), and a final exam worth 150 points will be given. Make-up quizzes and make up exam will be given for those with excused absences. Only two make-up quizzes and one make-up exam will be allowed. Make-up exam has to be done within one week of original scheduled test day. Excused absences are defined as documented illnesses, military service requirements, and family emergencies. All excused absences must be approved by the course instructor. ATTENDANCE You are expected to attend class regularly and punctually. All students are responsible for all information and materials given in class whether you are present or not. Excessive absences will be reported to the financial aid office and your academic advisor. Attendance at the laboratory is required and will be checked for each lab. Only one make-up lab is allowed with good excuses as mentioned under “Exams and quizzes.” Please turn off cell phones and beepers while in class out of consideration for your classmates. It can be very distracting- especially during an exam! Also, class time is not a social hour; please refrain from casual conversation during class time. 2 LECTURE OUTLINE Week Title of Chapter Concepts Covered 1 Chapter 10: 1/19-1/22 Introduction to Organic Chemistry Bonding, hybridization, shapes of molecules, bond angle, bond lengths, structural formula, common functional groups. 2 Chapter 11: Alkanes Introduction to alkanes and cycloalkanes, systematic nomenclature, conformations of alkanes and cycloalkanes, normal alkanes versus branched alkanes. Chapter 12: Alkenes Systematic nomenclature of alkenes and alkynes, structures of alkenes and alkynes, physical properties of alkenes and alkynes. 1/25-1/29 3 2/1-2/5 4 2/8-2/12 and Alkynes Chapter 12: Alkenes and Alkynes characteristic reactions of alkenes, important polymerization reactions of ethylene and substituted ethylenes. cont 5 2/15-2/19 6 2/22-2/26 7 2/29-3/4 Chapter 13: Benzene and its Derivatives Chapter 14: Alcohols, Structures, names, and physical properties of alcohols, Ethers, and Thiols characteristic reactions of alcohols, structures, names, and properties of ethers. Chapter 14: Alcohols, structures, names, and properties of thiols , important Ethers, and Thiols alcohols. cont Chapter 15: Stereochemistry 8 3/7-3/11 Introduction to benzene, systematic nomenclature, structure of benzene, characteristic reaction of benzene and its derivatives, phenols. Chapter 15: Stereochemistry Cis-trans isomers, diastereomers, chirality, enatiomers, optical activity, biology and chirality. Naming enantiomers, RS nomenclature; Racemic mixture, meso compound, composition, Fischer projection formulas. cont 9 Chapter 16: 3/21-3/25 Amines Structures, names, and physical properties of amines, the basicity of amines, the characteristic reactions of amines. 3 10 Chapter 17: 3/28-4/1 Aldehydes and Ketones 11 Chapter 18: 4/4-4/8 12 4/11-4/15 13 4/18-4/22 Carboxylic Acids Chapter 19: Carboxylic Anhydrides, Esters, and Amides Chapter 19: Carboxylic Anhydrides, Esters, and Amides cont 14 Chapter 20: 4/25-4/29 Carbohydrates 15 Chapter 21-22: 5/2-5/6 Lipids; Proteins Systematic nomenclature of name aldehydes and ketones, structures and properties of aldehydes and ketones, characteristic reactions of aldehydes and ketones, Keto-Enol Tautomerism. Systematic nomenclature of name carboxylic acids, structures and properties of carboxylic acids, characteristic reactions of carboxylic acids, soaps and detergents. Naming carboxylic anhydrides, esters, and amides ; preparing carboxylic anhydrides, esters, and amides; Characteristic reactions of anhydrides, esters, and amides; phosphoric anhydrides and phosphoric esters; step-growth polymerization. Introduction of carbohydrates; Fischer projections of monosaccharides; Haworth structures of monosaccharides; Chemical properties of monosaccharides; disaccharides; polysaccharides. Introduction to Lipids, Fatty acids, Waxes, Fats, and oils; chemical properties of triacylglycerols; gylcerophospholipids; steroids; cell membrane. Classify proteins, the name of an amino acid and its ionized structure, isoelectric point (pI), Identify the structural levels of a protein, name of a dipeptide, role of an enzyme in an enzyme-catalyzed reaction. 16 Finals Week 10:00-11:50 am, Wednesday, 5/11/2015 5/9-5/13 4 Tentative Schedule of Exams and Quizzes: CHEM 102 Assessment Week Quiz 1 (chpt 10) 2 Quiz 2 (chpt 11) 3 Quiz 3 (chpt 12) 4 Exam 1 (chpt 10-12) 4 Quiz 4 (chpt 13) 5 Quiz 5 (chpt 14) 6 Quiz 6 (chpt 15) 7 Exam 2 (chpt 13-15) 8 Quiz 7 (chpt 16) 9 Quiz 8 (chpt 17) 10 Exam 3 (chpt 16-17) 11 Quiz 9 (chpt 18) 12 Quiz 10 (chpt 19) 13 Exam 4 (chpt 18-19) 15 Homework: Since critical thinking and problem solving are important components of chemistry, homework will be assigned regularly and posted in WesternOnline in order to help students grasp principles and concepts discussed in class. Regular practice will help your overall exam scores. Web Resources: The course homepage is on the Western Online. Information for this class will be posted there, including the course syllabus, homework problem sets, quizzes, exams, their answers and your grades. Any class announcements will also be posted here. Emergency evacuation: If a fire alarm should happen to ring, or if students are ordered to evacuate a lab or classroom by the instructor, the students should walk to the nearest stairwell (Do not use the elevators) and proceed to the ground floor and out the building. Any student on an upper floor who cannot physically proceed down the stairs should go to the southernmost stairwell and await assistance. If the building should be evacuated all students and personnel 5 should gather at the southwest corner of the Higgins Parking lot near the fence (parking lot just outside the building) to wait further instructions. Also, the campus emergency management office has two videos at the beginning of the semester. These videos are titled "Shots fired and College Fire Survival" and can be found at the following web site: http://www.wiu.edu/rmep/resources.php. WIU Policies: It is the policy of Western Illinois University to accommodate individuals with disabilities pursuant to federal law and the University's commitment to equal educational opportunities. It is the responsibility of the student to inform the instructor of any necessary accommodations at the beginning of the course. Any student with a disability requiring accommodations should contact the Office of Disability Support Services. STATE ACCREDITATION POLICY INFORMATION: “In accordance with Illinois State Board of Education certification rules, all candidates seeking teacher certification are required by Western Illinois University to obtain a grade of “C” or better in all directed general education course, all core courses, and all courses in the option. Note: A “C- ” is below a “C”.” Please note: any secondary science teacher certification student wanting to see how this course is aligned with the State and National Standards should see their advisor and/or examine the Secondary Science Teacher Certification WesternOnline Advising site. Chemistry Resource Center. Chemistry resource center is located in Currens 107. Free tutoring and/or help is provided by the department through the Chemistry Help Center. Hours will be posted. Students with disabilities : In accordance with University values and disability law, students with disabilities may request academic accommodations where there are aspects of a course that result in barriers to inclusion or accurate assessment of achievement. To file an official request for disability-related accommodations, please contact the Disability Resource Center at 309-2982512, disability@wiu.edu or in 143 Memorial Hall. Please notify the instructor as soon as possible to ensure that this course is accessible to you in a timely manner. 6 Chem 102 Lab Syllabus Time: Tuesday: 8-10:50 am; 11:00-1:50 pm; 2-4:50 pm. Thursday: 8-10:50 am; 11:00-1:50 pm; Place: CURRENS HALL 427 Lab Manual: Chemistry 102 Lab Experiments; Sub Title 1: Western Illinois University; by Bettelheim and Landesberg; Cengage; Custom Lab Manual ISBN-9781337048231 Experiments: Each student is expected to successfully complete 10 experiments. Lab Reports: Laboratory reports will consist of three parts: pre-lab questions, report sheet and post-lab questions. The report should be hand written legibly. Each complete lab report is 20 points. Pre-lab questions (5 pts) : The pre-lab questions are to be completed before coming to lab. Your instructor will check your pre-lab report at the beginning of each laboratory period. One will not be permitted to perform an experiment without completing the pre-lab section of the lab report before the start of the lab. Report sheet (10 pts): Report sheets include experimental data, calculations and observations. Report sheet should be recorded during the lab. Post-lab questions (5 pts): All questions should be answered unless otherwise indicated by your instructor. Lab report should be submitted at the beginning of the next lab period. Late lab reports will be docked by 3 pts per day. Lab reports turned in one week after the due date will not be graded. Attendance: Attendance is required. One and only one make-up lab is allowed at the end of the semester. You must attend the lab section that you are signed-up for. Cell phones are not to be used in the lab, and should be turned off and put away during lab period. Grading: The instructors will collaborate so as to grade each lab section in a uniform manner. Overall, the lab will count as 250 points and it will be added to your lecture portion points to tabulate a final grade for this course. Failure to complete the lab with at least 60% (>150 points out of 250 lab grade) will result in failure for the Lab. Since the laboratory experience is integral to the overall course, failure to earn a passing grade in the lab will result in an automatic failure for the course. Statement on Ethics. Western Illinois University, like all communities, functions best when its members treat one another with honesty, fairness, respect, and trust. Students have rights and responsibilities. The following action is prohibited under the Student Conduct Code: Disorderly Conduct: Any behavior which disrupts the regular or normal functions of the University community, including behavior which breaches the peace or violates the rights of others. Plagiarism, cheating, and other forms of academic dishonesty constitute a serious violation of University conduct regulations. Any student convicted of academic dishonesty, can receive a failing grade and may be subject to further academic penalties. Web address for Academic Integrity Policy (http://www.wiu.edu/policies/acintegrity.php). Please remember that you are expected to do your own work at all times. You can’t directly copy the homework or lab report from your 7 classmates or other sources. However, discussing homework questions or lab reports with your classmates and others is fine. Students are expected to wear clothing that completely covers the feet and legs in lab and wear safety goggle all the time in the lab. Be aware, sandals and shorts are not appropriate dress in lab. Students who wear sandals or shorts will not be allowed to work in the lab. Supplies to be provided by students - Safety goggles. Eye protection is required and must be purchased by the student. - Scientific Calculator (not a graphing calculator) Schedule of Experiments Week Activities Points 1 2 Lab # - No lab Lab check-in (Lab safety rules) 5 3 21 Structure in Organic Compounds(Use of Molecular Models I): To visualize structure in organic molecules, build and compare isomers, explore the three-dimensional character. 20 4 24 5 23 6 25 7 22 8 9 28 10 26 Classification and Identification of Hydrocarbons: To investigate the physical properties, solubility, and density of hydrocarbons, to compare the chemical reactivity of an alkane, an alkene, an aromatic compound. Column and Paper Chromatography(Separation of Plant Pigments): To compare separation of components of a mixture by two different techniques, Classification and Identification of Alcohols and Phenols: To learn characteristic reactions of alcohols and phenols, identification of an unknown compound(an alcohol or a phenol). Stereochemistry(Use of Molecular Models II): To study the conformation of cyclohexane, to distinguish between chiral and achiral systems, to define and illustrate enantiomers, diastereomers, and meso forms. 20 Lab Midterm Properties of Amines and Amides: To show some physical properties of amines and amides, to demonstrate the hydrolysis of amides. Classification and Identification of Aldehydes and Ketones: To learn the chemical characteristics of aldehydes and ketones, to distinguish between examples of aldehydes and ketones, identify 20 20 20 20 20 20 8 11 27 12 30 13 31 14 15 16 34 - aldehydes and ketones. Properties of Carboxylic Acids and Esters: To study the physical properties of carboxylic acids, to prepare a variety of esters and note their odors, to demonstrate saponification. Preparation of Acetylsalicylic Acid (aspirin): To illustrate the synthesis of the drug aspirin. Isolation of Caffeine from Tea Leaves: To demonstrate the isolation of natural product, to learn the techniques of extraction. Thanksgiving break Make-up lab Lab check-out Lab final (comprehensive) 20 20 20 5 20 9