Reducing CO to Dense Nanoporous Graphene by Mg/Zn for High Power Electrochemical Capacitors
advertisement

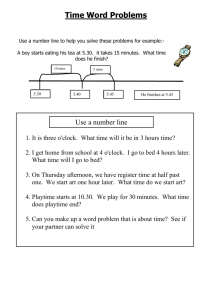
Reducing CO2 to Dense Nanoporous Graphene by Mg/Zn for High Power Electrochemical Capacitors Zhenyu Xing,a† Bao Wang,a† Wenyang Gao,e Changqing Pan,c Joshua K. Halsted,a Elliot S. Chong,a Jun Lu,b Xingfeng Wang,a Luo Wei,a Chih-Hung Chang,c Youhai Wen,d Shengqian Ma,e Khalil Amineb,* and Xiulei J i a,* a Department of Chemistry, Oregon State University, Corvallis, Oregon 97331 United States b c Argonne National Laboratory, Lemont, Illinois 60439, United States School of Chemical, Biological and Environmental Engineering, Oregon State University, Corvallis, Oregon 97331, United States d National Energy Technology Laboratory, Albany, Oregon 97321, United States e Department of Chemistry, Tampa, Florida 33620, United States † These authors equally contributed to the paper. E-mail: david.ji@oregonstate.edu amine@anl.gov 1 Figure S1. XRD patterns of the products after CO2 reduction by Mg as a reductant mixed with different metal carbonates. a, A mixture of Mg and SrCO3. Patterns show that both SrCO3 and SrO coexist, indicating that the reaction temperature is above 1100 °C, the decomposition temperature of SrCO3. b, A mixture of Mg and BaCO3. BaO is not detectable in the XRD Pattern after the reaction, suggesting that the reaction temperature is below 1450 °C, the decomposition temperature of BaCO3. 2 Figure S2. N2 sorption isotherms and pore size distribution of C-Ms (a to k) and C-MZ-n (l to r) formed at different conditions. a, 650 °C, 70 CCM for 60 mins. b, 680 °C, 70 CCM for 60 mins. c, 710 °C, 70 CCM for 60 mins. d, 740 °C, 70 CCM for 60 mins. e, 680 °C, 70 CCM for 5 mins. f, 680 °C, 70 CCM for 15 mins. g, 680 °C, 70 CCM for 30 mins. h, 680 °C, 70 CCM for 45 mins. i, 680 °C, 70 CCM for 90 mins. j, 680 °C, 26 CCM for 90 mins. k, 680 °C, 229 CCM for 90 mins. i, 680 °C, 26 CCM for 90 mins. l, 680 °C, 70 CCM for 60 mins, Zn/Mg = 0.5. m, 680 °C, 70 CCM for 60 mins, Zn/Mg = 1. n, 680 °C, 70 CCM for 60 mins, Zn/Mg = 2. o, 680 °C, 70 CCM for 60 mins, Zn/Mg = 3. p, 680 °C, 70 CCM for 60 mins, Zn/Mg = 4. q, 680 °C, 70 CCM for 60 mins, Zn/Mg = 5. r, 680 °C, 70 CCM for 60 mins, Zn/Mg = 6. a b 3 c d e 4 f g h 5 i j k 6 l m n 7 o p q 8 r 9 Figure S3. XRD pattern of the product after the reaction between Zinc and CO 2 at 710 °C. The product is lightly yellowish. No black carbon appears to be formed during the reaction. 10 Figure S4. TEM images. a, A representative image for amorpnous nanoporous carbon. b, A TEM image of C-MZ-3, demonstrating the sharp structural contrast between amorphous nanoporous cabron and few-layered graphene constructed nanoporous graphene. 11 Figure S5. TEM images of product of CO2 reduction by pure Mg before HCl etching demonstrating homogeneous distribution of MgO in G-M and supporting that porosity in C-M is generated by removal of MgO. a, HAADF Scanning TEM of C-M before HCl etching. b,c,d, Energy dispersive X-ray elemental mappings of C, Mg and O, respectively. 12 Figure S6. TEM images of product of CO2 reduction by a mixture of Zn/Mg with an atomic ratio of 3 before HCl etching, demonstrating homogeneous distribution of MgO and ZnO in G-MZ and supporting that porosity in C-MZ is generated by removal of MgO and ZnO. a, HAADF Scanning TEM of C-MZ before HCl etching. b,c,d, Energy dispersive X-ray elemental mappings of C, Mg and Zn, respectively. 13 Figure S7. Electrochemical characterizations of C-M formed by the reduction of CO2 by Mg at 680 °C for one hour under a CO2 flow rate of 70 CCM. a, CV curves at different sweeping rates. The well-maintained rectangular-shape CV at very high sweeping rates demonstrates the high rate capability. b, CD curves at 5 A/g and 10 A/g. c, EIS results and equivalent circuit simulation (inset), d, Specific capacitance as a function of ac current frequency. A capacitance of 3 F/g is maintained at a high ac frequency of 120 Hz. 14 Figure S8. Galvanostatic charge/discharge cycling performanc and the corresponding coulombic efficiency of the two-electrode cell with C-MZ as the electrdoe at a current rate of 10 A/g. 15 Figure S9. SEM images of as-formed porous graphenes demonstrate the bulk morphology. a, C-M prepared at 680 °C and a CO2 flow of 70 CCM in 60 mins. b, C-MZ3. 16 Figure S10. Bode plots with phase angle as a function of frequency. a, C-M prepared at 680 °C and a CO2 flow of 70 CCM in 60 mins. b, C-MZ-3. 17