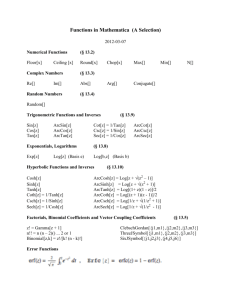
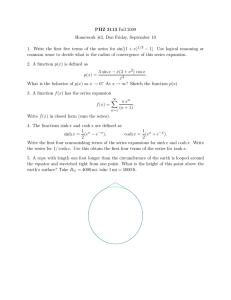
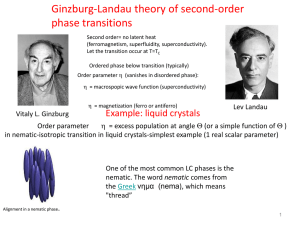
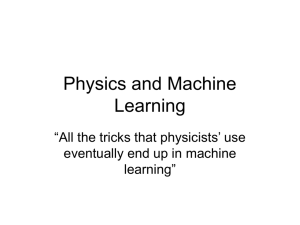
Course 443, Problem Set, Michaelmas Term, 2003
advertisement

Course 443, Problem Set, Michaelmas Term, 2003
Show that the Kelvin-Planck and Clausius statements of the
second law of thermodynamics are equivalent
Suppose Kelvin-Planck untrue:
There exists an engine E, that takes heat Q1 from hot body and delivers
W = Q1 . This drives a second engine R, which also extracts heat Q2 from
a cold body. The heat delivered by R is Q1 + Q2 . R extracts heat Q2 and
delivers net heat (Q1 + Q2 ) − Q1 = Q2 , with no work done. This violates the
Clausius statement.
Suppose Clausius untrue:
There exists and engine R that extracts Q2 from cold body and delivers
Q2 to a hot body with no work. Consider an engine E that extracts Q1 from
the hot body and deliveer Q2 to the cold body with work done W = Q1 − Q2 .
The composite engine takes in heat Q1 − Q2 and does W = Q1 − Q2 . This
violates the Kelvin-Planck statement.
1
Show that cP − cV = R for an ideal gas in classical thermodynamics
∂U
|V
∂T
and for an ideal gas U = U (T ) so that
cV =
dU
|V
dT
⇒ dU = cV dT
1st law
⇒ dQ = cV dT + P dV
cV
=
The specific heat at constant pressure is (using dQ = cP dT )
cP dT = cV dT + P dV
dV
⇒ cP = cV + P
|P
dT
Then
P V = nRT
∂V
P
|P = nR
∂T
⇒ cP − cV = nR
2
(1)
Determine the chemical potential, µ(T, p, c) where c = N/V for a
perfect gas in the canonical ensemble
Starting from
1 Vg N
Z=
N ! λ3
Then
1
F = − ln Z
β
!
1 Vg N
1
ln
= −
β
N ! λ3
1
1
Vg
ln
= −
+ ln
β
N!
λ3
√
Vg
1
−ln 2π − N (ln N − 1) + N ln 3
= −
β
λ
!
3
√
Nλ
1
N ln
=
− N + ln 2π
β
Vg
!
√
∂F
1 ∂
N λ3
=
− N + ln 2π ‡
N ln
∂N
β ∂N
Vg
3
1 Nλ
ln
=
β
Vg
† using N ! =
√
2πN N e−N and ‡ using ln ax =
µ(T, P, ci ) =
d
(x ln ax − x). Then
dx
1 N λ3
1 Ni N kT λ3
ln
= ln
β
Vg
β N V gkT
and
µ(T, P, ci ) =
as required.
3
†
1 cP λ3
ln
β
gkT
Compute the two-point correlation function
hsi sj i = Z −1
X
{si }
si sj exp (−βH[{si }])
for the 1D Ising model with periodic boundary conditions.
Assuming periodic boundary conditions and no external field
E=−
Then
hsk sk+1 i =
where
N
−1
X
Ji si si+1
i=1
PN −1
X
1 X
sk sk+1 e i=1 βJi si si+1
...
ZN s1 =±1 sN =±1
ZN = 2
NY
−1
2 cosh βJi
i=1
Note that the derivative the exponential w.r.t. Jk brings down a factor sk sk+1 .
Using this result we consider the nearest neighbour correlation function (with
r = 1) and assume that Ji = J. Then
hsk sk+1 i =
=
P
X
1 X
sk sk+1 e βJi si si+1
...
ZN s1 =±1 sN =±1
X P
1 1 ∂ X
...
e βJi si si+1
ZN β ∂Jk s1 =±1 sN =±1
1 1 ∂
[ZN (J1 , . . . , JN −1 ] |Ji =J
ZN β ∂Jk
−1
NY
−1
1 NY
2 sinh βJ
= 2
2 cosh βJ 2
β i=1
i=1
= tanh βJ
=
For G(r = 2) use s2k+1 = 1 to write sk sk+2 = sk sk+1 sk+1 sk+2 and as above
PN −1
1 X
sk sk+1 sk+1 sk+2 e i=1 βJi si si+1
ZN
1 1 ∂ 2 ZN (J1 , . . . , JN −1 )
=
ZN β 2
∂Jk Jk+1
2
= tanh βJ
G(r = 2) =
4
Therefore for arbitrary separation, r
1 1 ∂
∂
∂
...
ZN
r
ZN β ∂Jk ∂Jk+1
∂Jk+r−1
= tanh βJk tanh βJk+1 . . . tanh βJk+r−1
G(r) =
=
r
Y
tanh βJk+r−1
k=1
= (tanh βJ)r
if Ji = J.
5