Hypopthalmichthys nobilis) Katelin P. Cross Abstract:
advertisement

Katelin Cross
Bighead Carp (Hypopthalmichthys nobilis)
Katelin P. Cross
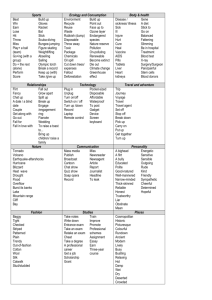
Figure 1.1
Image of non-native Bighead Carp (Hypopthalmichthys nobilis) taken from the U.S. Fish and Wildlife website,
http://www.fws.gov/.
Abstract:
The Bighead Carp, Hypopthalmichthys nobilis, is a large cyprinid fish native to China. It has
been introduced to areas of Europe and North America as a food fish for human consumption
and to control eutrophication processes. It filters the water with its gill rakers removing large
algae and zooplankton from the water column. Consumption of zooplankton causes a negative
effect on native species that compete for the same resource, and reduction of zooplankton causes
an increase of algal blooms that fish farmers want to control.
Taxonomy:
Phylum Chordata, Subphylum Vertebrata, Superclass Gnathostomata, Grade Pisces, Subgrade
Teleostomi, Class Osteichthyes, Subclass Actinopterygii, Infraclass Neopterygii, Division
Halecostomi, Subdivision Teleostei, Superorder Ostariophysi, Series Otophysi, Order
Katelin Cross
Cypriniformes, Suborder Cyprinoidei, Subfamily Hypophthalmichthyinae (Jennings 1988), and
the Genus and Species Hypopthalmichthys nobilis.
Order: Cypriniformes is the largest order of fish. There are more than 3200 species listed in this
order. Cyprinids are found in abundance in Southeast Asia, Europe, Africa and North America.
Cyprinids have pharyngeal dentition, which are tooth-bearing pharyngeal arches located in the
throat region, and a highly protrusible upper jaw (Helfman 2009).
Family: Cyprinidae is one of the largest vertebrate families with 1700 species. The majority of
the Cyprinids are quite small and tend to be prey for piscivorous fishes. Minnows are either
insectivorous or herbivores. Minnows produce an alarm substance in their skin that is only
released when the skin is broken. It warns other fish of attack and attracts other predators (Ross
2001).
Characteristics:
Bighead Carp is large, oblong and laterally compressed with a large, terminal mouth (Ross
2001). The head and mouth are large compared to the body. The small eyes are located low on
the head close to the angle of the mouth. Lips are thin with no labial fold (Jennings 1988). The
gill rakers are long and closely arranged to filter fine food particles (Berry and Low 1970).
Bighead Carp has small cycloid scales and a complete lateral line. Lateral line has 85-100 scales
with 26-28 scale rows above and 16-19 scale rows below (Ross 2001). Dorsal fin has less than 9
branched rays, and dorsal origin is posterior to pelvic fin base (Jennings 1988). The Bighead
Carp has 13-15 anal rays, 16-21 pectoral rays, and 9-10 pelvic rays. The abdominal keels are
prominent, and the ventral profile is more convex than the dorsal profile (Berry and Low 1970).
Katelin Cross
The head and top of the carp are an olive to dark gray switching to silvery to a yellowish-white
on the sides and abdomen. The body is mottled with numerous irregular, dark gray to black
blotches. The fins are a dusky brown (Ross 2001).
Distribution:
The Bighead Carp is native to eastern China in the lowland rivers of the north China plain and
south China. It has been distributed to at least 32 countries for use in aquaculture and/or human
consumption. The species was first introduced to the United States in Arkansas in 1972 by a
private fish farmer to improve the water quality of his fish ponds. Bighead Carp can now be
found in open waters in the United States (Jennings 1988). It is thought that specimens had
escaped the fish ponds and are now well established. In Mississippi, Bighead Carp have been
documented in the Pascagoula drainage, the Mississippi River and the Yazoo River. The
Bighead Carp is also being raised in at least nine fish farms within Mississippi. Bighead Carp
became more abundant in Mississippi’s delta rivers after the flood of 1991 (Ross 2001).
Form and Function:
Pharyngeal teeth are 0,4-4,0 (Ross 2001). The fifth brachial arch is modified so that each arch
bears one or more rows of uniserially arranged, laterally compressed teeth (Berry and Low
1970). Bighead carp uses the gill rakers to filter the water column for large algae and detritus
particles (Opuszynski and Shireman 1991). Their diet makes this species desirable for the
prevention of intense blooms of blue-green algae and other phytoplankton (Jennings 1988). The
gill rakers consist of a pliable and soft supporting skeleton and are covered in a thin layer of
mucus. The esophagus is short and more or less cylindrical. Cyprinoid fishes lack a stomach
proper, and the pyloric sphincter separates the esophagus from the intestinal swelling, the typical
Katelin Cross
sac-like region which represents the stomach (Berry and Low 1970). The entire digestive tract is
3.17-5.01 times the total body length. The swim bladder is divided into two chambers and lie
between the kidneys and the gut (Jennings 1988).
Ontogeny and Reproduction:
Spawning occurs from April to June, peaking in late May. Water temperatures must be greater
than 18ºC for spawning. Adults migrate out of river channels when the water level rises and into
rapidly flowing waters. The promiscuous male will actively chase females and occasionally prod
the abdomen of the females with their heads. This prodding action typically occurs at the surface
of the water. Like most fish species, the Bighead Carp reproduces by external fertilization.
Fertilization is also monospermic and occurs in the egg at the metaphase of the second
maturation division. 1 mL of milt contains about 48 million spermatozoa. Spawning can occur
more than once a year. After spawning occurs, the adult Bighead carp will then migrate to
floodland lakes (Jennings 1988).
The fertilized eggs are deposited behind sandbars, among rocks of the rapids in river channels,
and at islands formed by junctions of currents. The eggs are nonadhesive and bathypelagic
meaning they must float to hatch (Jennings 1988). The current of the water must be at least 80
cm/s to keep them in suspension. Eggs will hatch about one day after fertilization when the water
temperature is around 22-26ºC. The larvae and juveniles use the floodplains as a nursery (Ross
2001).
Newly fertilized eggs are 1.4-1.5 mm in diameter that will swell to 4.7-5.2 mm in diameter with
an uptake of water. Protolarvae at hatching are 5.5-6.0 mm in total length. Protolarvae have 2426 preanal and 15-19 postanal myomeres. 3 days after hatching, larvae are about 8.5 mm long,
Katelin Cross
and a movable gill-jaw apparatus develops and starts to function. A rudimentary swimbladder is
functioning and allowing the larvae to actively move in the water column. About 4.5-5 days
post-hatching, the swimbladder is fully functioning and respiration occurs exclusively by the
gills. The larvae are 8.5 to 9 mm in length at this point. After one week from hatching, the yolk
sac is completely resorbed and active feeding occurs. When the juveniles of the Bighead Carp
are one month old, small scales will start to develop along the mid-line of the body and will
completely cover the body when the juveniles are about 1.5 months old (Jennings 1988). Male
Bighead Carp will reach sexual maturity in at least 3 years. The females will reach sexual
maturity between 4 to 5 years (Chen et al. 2007). In the United States, the Bighead Carp will
reach a maximum weight of 18 to 23 kg in about 4 to 5 years (Jennings 1988).
Ecology:
Population Characteristics- In a spawning site, there are 2 to 3 male Bighead Carp for every
female. Sexual maturity based on age is dependent upon abiotic factors like the environment and
climate conditions. Males will reach sexual maturity before the females (Chen et al. 2007).
Maximum longevity is at least nine years. The Bighead Carp is becoming more and more
common throughout its locations and is being increasingly caught more by fishermen (Jennings
1988).
Space Use- Bighead Carp are found in the pools of large rivers. This species has a tolerant of
brackish water and can survive in water with salinities up to 12 ppt (Ross 2001). The furthest this
species has been successfully acclimatized is 45ºN in the Soviet Union. Bighead carp fry are
captured at the surface of the water. Adults are found at a slightly lower depth (Jennings 1988).
Katelin Cross
Diet- Bighead carp are planktivores. They feed by using their gill rakers to filter zooplankton
and phytoplankton from the water (Radke 2005). They also filter detritus particles out of the
water column (Opusynski and Shireman 1991). When zooplankton biomass is low, the Bighead
Carp will switch to primarily consuming phytoplankton including blue-green algae and detritus.
Larvae of Bighead Carp at 7-9 mm long will consume primarily protozoa and small zooplankton
including copepodites, rotifer and nauplii. At 10-17 mm, the larvae will start to consume
cladocera, and at 18-23 mm, they will start to consume phytoplankton. Once they reach the
lengths of 24-30 mm, the larvae form of Bighead Carp will readily consume both zooplankton
and phytoplankton (Jennings 1988).
Research was conducted to observe the impacts of Chinese carps on plankton communities of
channel catfish ponds. The results showed that the carps fail to control algae because they
primarily consume zooplankton. The reduction of zooplankton causes an algal bloom that the
fish farmers were trying to control when they stocked their ponds with silver and bighead carp
(Burke et al. 1986). Not only do they negatively affect ponds that they were distributed to when
the zooplankton is abundant, they negatively affect the native species of the water systems they
invaded. A study conducted by Schrank, Guy and Fairchild suggested that the Bighead Carp has
the potential to negatively affect the growth of the American Paddlefish when competing for
food resources (2003). Both Bighead Carp and the American Paddlefish are planktivores with
similar spacing of the gill rakers. This causes both species to consume similar size copepods,
insect larvae and amphipods. Interspecific competition occurs when the zooplankton is limited,
and the Bighead Carp is better at filtering out the food because of the mucus on its gill rakers
which causes a decrease in food and body mass for the American Paddlefish. Also, the Bighead
Carp has an ecological advantage over the American Paddlefish by being able to switch to
Katelin Cross
smaller food resources when large zooplankton are limited due to the mucus on the gill rakers of
the Bighead Carp. It is theorized that the Bighead Carp show aggressive behavior to the
American Paddlefish preventing it from consuming zooplankton ( Schrank et al. 2003).
Behavior:
Any forms of migration of the Bighead Carp are usually caused by the need to find faster
flowing waters for reproduction or in search of more food (Jennings 1988). Bighead Carp have
been described as a “quiet schooling fish, easily caught from lakes and reservoirs" (Vinogradov
1979).
Genetics:
Bighead Carp have 48 diploid chromosomes, and all chromosomes in the karyotype have a
homologous pair. The karyotype has 84 chromosome arms with 20 metacentric, 16
submetacentric, and 12 telocentric chromosomes. Chromosome length varied from 1.8 to 4.98
µm. The total length of the chromosome set was equal to 73.9 µm (Marian and Krasznai 1979).
Conservation:
Bighead Carp is an exotic species that has established in Mississippi’s waterways (Ross 2001).
Bighead Carp in China are fished for during their reproductive season which is May to June.
The flesh is palatable, although bony (Jennings 1988).
Acknowledgments:
Katelin Cross
I want to thank Dr. Eric Dibble and Clint Lloyd for assistance with my research and paper, and I
want to heavily thank all the peer reviewers especially Chazz Coleman that helped me
understand how a scientific paper is supposed to be formatted. I also want to thank the staff at
Mitchell Memorial Library with helping me find the papers I need to properly write this paper.
Without all of you, my paper will not succeed.
Remarks:
Bighead Carp is sometimes referred to as marbled carp or speckled carp (Ross 2001). Jennings
noted that 4 scientific synonyms: Leucisus nobilis (Canton), Cephalus hypopthalmus (Hong
Kong), Hypopthalmichthys mandschuricus (Shanghai), Hypophthalmichthys simoni (Yangtze
Kiang) (1988). Hypopthalmichthys nobilis is known as Aristichthys nobilis based upon the
divergent gill raker form, pharyngeal dentition and abdominal keel length (Ross 2001).
Literature Cited:
Berry, P. Y., and M. P. Low. 1970. Comparative studies on some aspects of the morphology and
histology of Ctenopharyngodon idellus, Aristichthys nobilis and their hybrid. Copeia
1970:708-726.
Burke, J.S., D.R. Bayne and H. Rea. 1986. Impact of Silver and Bighead Carps on plankton
communities of Channel Catfish ponds. Aquaculture 55:59-68.
Chen, P., E.O. Wiley and K.M. Mcnyset. 2007. Ecological niche modeling as a predictive tool:
silver and bighead carps in North America.
Helfman, G. S. 2009. Chapter title in Teleosts at last 1: bonytongues through anglerfishes:
The diversity of fishes. New Jersey: Blackwell Publishing. 269
Katelin Cross
Jennings, Dawn P. 1988. Bighead carp {Hypophthalmichthys nobilis): a biological synopsis.
U.S. Fish Wild. Serv., Biol. Rep. 88(29).35 pp.
Marian, T., and Z. Krasznai. 1979. Comparative karyological studies on Chinese carps.
Aquaculture 18(4): 325-336.
Opuszynski, K., and J.V. Shireman. 1991. Food passage time and daily ration of bighead carp,
Aristichthys nobilis, kept in cages. Environmental Biology of Fishes. 30:387-393.
Radke, R. 2005. Issg Database: ecology of Hypopthalmichthys nobilis. Issg Database: Ecology of
Hypopthalmichthys nobilis.
Ross, S. 2001. “Hypopthalmichthys nobilis (Richardson), Bighead Carp.” The Inland Fishes of
Mississippi. 162-64.
Sally J. Schrank , Christopher S. Guy & James F. Fairchild (2003): Competitive Interactions
between Age-0 Bighead Carp and Paddlefish, Transactions of the American Fisheries
Society, 132:6, 1222-1228
Vinogradov, V. K. 1979. Herbivorous fish breeding and rearing. In E. A. Huisman and H.
Hogendoorn, eds. EIFAC Workshop on mass rearing of fry and fingerlings of freshwater
fishes/papers 8-11 May 1979. EIFAC Tech. Pap. 35 (Suppl. 1):106-113.