Physics 362, States of Matter, Spring 2014 Physics 362 States of Matter
advertisement

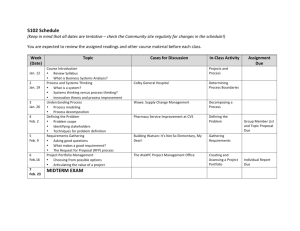
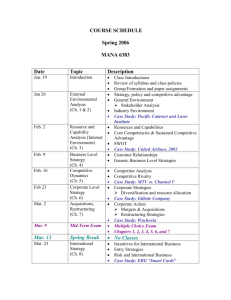
Physics 362, States of Matter, Spring 2014 1 Physics 362 States of Matter Spring, 2014 at Hope College Instructor: Steve Remillard Office: VanderWerf 205 Phone: 395-7507 Office Hours: M,R 16:00-16:50; F 9:30-10:20 Email: remillard@hope.edu Lecture: M,W,F 14:00-14:50 R 11:00-11:50 Prerequisite Phys 270 Modern Physics, MATH 232 Multi II, PHYS 280 Math Phys. (co-req) Overview of this Subject States of Matter is concerned with the collective behavior of large ensembles of particles. Whether the subject at hand is superfluid liquid helium, the free energy of a superconductor, radiation pressure inside a collapsing star, cooling by adiabatic demagnetization, or the macroscopic occupation of the ground state in a Bose-Einstein condensate, the principles in this course are the gatekeeper to their exploration. Another name for this course could be Thermodynamics and Statistical Mechanics. These two interwoven subjects are hard to separate. For example, temperature is a measure of how stable the internal energy is against variations in entropy, and entropy is arrived at statistically. It is assumed that you have already studied and mastered fundamental thermodynamics. This includes such pedestrian concerns as the first law of thermodynamics, as well as applications of the ideal gas law to gas processes and cycles. It is assumed that you have already studied and mastered quantum distributions, such as the Fermi-Dirac distribution. This includes the elementary use of Fermi-Dirac statistics to determine how many of the states in the density of states function are actually occupied. You are now at a level where, hopefully to your delight, much deeper understanding is possible. In more elementary courses you were asked to believe the ideal gas law. In this course you will understand why PV sometimes equals nRT, and why often times it doesn’t. You were perhaps told once that rate constants increase with temperature. In your textbook, you will find the derivation of the van’t Hoff equation which ensures that this is so. In more elementary courses you are told that radiation transfers heat at a rate that is quartic in the temperature. In this course you will tear in to the statistical mechanics of photons, the machinery that provides us with this piece of very useful common knowledge. I look forward to this semester with you. I hope that you will conclude, as I did long ago, that States of Matter is the right of passage to becoming a physicist. Text for this course Concepts in Thermal Physics, 2nd Ed. by Stephen J. Blundell and Katherine M. Blundell (Oxford University Press. 2010), ISBN 978-0-19-956210-7 Why I chose this book: My choice is predicated on the extremely clear explanations, and also on the several topical chapters near the end, all of this presented in bite-sized chapters which break the monotony. The end-of-chapter problems are sparse, and many are trivial, so I have added problems that I would like to see in the book. Rev 1 Physics 362, States of Matter, Spring 2014 2 Another useful text at this level An Introduction to Thermal Physics, by Daniel V. Schroeder (Addison Wesley Longman, 2000), ISBN 0-201-38027-7 Course Evaluation Two tests (20% each) One cumulative final exam Homework 40% 30% 30% Grading System 93-100%=A, 90-93%=A–, 87-90%=B+, 83-87%=B, 80-83%=B–, 77-80%=C+, 73-77%=C, 7073%=C–, 67-70%=D+, 60-67%=D, Below 60%=F The Course Web Site: The Moodle site for this course is the only place where homework assignments will be found. Check there often. Do not print it out at the start of the semester assuming that all of the assignments have been posted. Rather, check each week for that week’s work. There will also be articles and useful downloads posted there, as well as some homework solutions. Homework o There will be one weekly assignment due every Friday at 5:00 pm. To accommodate holidays and tests, some assignments may be due on a different day than Friday. Consult the assignments page on the course web site. o The readings listed in the syllabus need to be finished prior to the indicated class date. This is the only way this course will work for you. Class time will build on the reading – not replace it. Quizzes might be necessary to force this practice. Tests & Exam Two, 50 minute long tests. Closed note and closed book. One 8.5x11 cheat sheet allowed. One, two hour long final exam. Open note and open book. Disabilities If you require accommodation for any kind of disability please contact me during the first week of the semester. Several useful services are also available from the Office of Disability Services (395-7805) and the Academic Support Center (395-7830). Withdrawing The deadline for withdrawing from this course or converting to P/F is March 12, 2014. Rev 1 Physics 362, States of Matter, Spring 2014 3 Course Schedule. Changes will be announced. Date Topic Overview of Heat and Thermostatistics Jan 7 Introduction to thermostatistics Jan 8 Heat Jan 9 Misc math topics Jan 12 Macrostates and Microstates Jan 14 Applications of the Boltzmann distribution The Kinetic Theory of Gases Jan 15 The Maxwell-Boltzmann distribution Jan 16 No Class, CUWIP Jan 19 Doppler broadening Jan 21 The kinetic theory of pressure and molecular effusion Jan 22 The kinetic theory of pressure and molecular effusion Jan 23 Mean free path The First Law of Thermodynamics Jan 26 Heat transfer Jan 28 Thermal diffusion in solids Jan 29 The fundamental theorem of calculus: path independence Jan 30 Thermal energy Feb 2 Heat capacity Feb 4 The equipartition theorem Feb 5 Gas processes Feb 6 Catch up or get ahead Feb 9 Winter Break The Second Law of Thermodynamics Feb 11 Thermal cycles Feb 12 Heat engines Feb 13 Entropy Feb 16 Entropy Thermodynamic energy potentials Feb 18 Thermodynamic potentials Feb 19 Thermodynamic potentials and Maxwell relations Feb 20 Paramagnetism and potentials Feb 23 Catch-up or get ahead The Development of Statistical mechanics Feb 25 The partition function Feb 26 TEST 1 Feb 27 The partition function No Class - March Meeting (Mar 2 & Mar 4 might switch) Mar 2 Mar 4 The statistical mechanics of ideal gases Mar 5 The statistical mechanics of ideal gases Mar 6 Relativistic effects Mar 9 Catch up or get ahead Rev 1 Reading Chapter 1 Chapter 2 Chapter 3 Chapter 4.1-4.6 Chapter 4.7 Chapter 5.1-5.2 Chapter 5.3 Chapter 6 Chapter 7 Chapter 8 Chapter 9.2, Handout Chapter 9.3-9.4 Appendix C.6-C.9 Chapter 11.1-2 Chapter 11.3 Chapter 19 Chapter 12 Chapter 13.1-13.5 Chapter 13.6-13.7 Chapter 14.1-5 Chapter 14.6-8 Chapter 16.1-5 Chapter 16.6-7 Chapter 17.3-4 Chapter 20.1-3 Chapters 1-19, not 10,15,18 Chapter 20.4 Chapter 21.1-4 Chapter 21.5-6 Chapter 25 Physics 362, States of Matter, Spring 2014 4 The Chemical Potential Mar 11 Adsorption and the chemical potential Mar 12 The grand partition function Mar 13 Spring Break Mar 20 Spring Break Mar 23 The grand potential; The grand canonical ensemble Mar 25 The law of mass action; Osmosis Non-ideal and Quantum Matter Mar 26 The statistical mechanics of photons Mar 27 Blackbody radiation Mar 30 Phonons – The Einstein model Apr 1 Phonons – The Debye model Apr 2 The van der Waals gas Apr 3 The Virial expansion Apr 6 Cooling real gases Apr 8 Throttling processes Apr 9 Phase transitions Apr 10 The Clausius-Clapeyron equation Apr 13 Higher order phase transitions Apr 15 Bose-Einstein and Fermi-Dirac distributions Apr 16 Catch-up or get ahead Apr 17 TEST 2 Apr 20 Fermi gases Apr 22 Bose Einstein condensation Apr 23 Nonequilibrium thermodynamics Apr 24 Thermoelectricity Apr 28 Exam, Tuesday at 12:30 Rev 1 Baierlein AmJPhys article Chapter 22.1-3 Chapter 22.4 Chapter 22.5-9 Chapter 23.1-5; App C.1-4 Chapter 23.6-8 Chapter 24.1 Chapter 24.2-24.3 Chapter 26.1-2 Chapter 26.3-4 Chapter 27:1-2 Chapter 27:3-4 Chapter 28.1-3 Chapter 28.4-6 Chapter 28.7-8 Chapter 29 Chapters 20-29 Chapter 30.1-3 Chapter 30.4 Chapter 34.1-3 Chapter 34.4-5