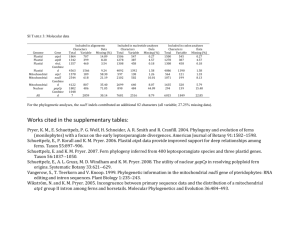
first individual to scan was the adult males present; dominant
advertisement

Current Biology Vol 16 No 17 R670 first individual to scan was the first to cross the small road in 100% of cases, compared to 70% for the large road. On the large road the second-ranking male sometimes continued scanning while the elderly third male and alpha female took up the lead on the large-road progressions. The alpha male increased his rearward presence on the large road, whereas the alpha female showed a dramatic reduction in frequency of being last; in other words when the degree of risk increased she took up a more forward position. Additionally, when the alpha male was present in mixedgroup progressions containing one other adult male (N = 6, mean group size: 6.7), he was first to scan and cross in 50% of large road- crossings and last in only 33%. This suggests that his rearward position at other times was not due to fear. Modern Bossou chimpanzees encounter predators infrequently [6], and although humans themselves are not ‘predators’ of these chimpanzees, we propose that road-crossing, a human- created challenge, presents a new situation that calls for flexibility of responses by chimpanzees to variations in perceived risk. Crossing the large road and leaving forest for open areas are potentially risky situations for chimpanzees, reflected in increased waiting time. During dangerous excursions certain positions may be more advantageous than others, depending upon age and sex [4]. Adult males, less fearful and more physically imposing than other group members, take up forward and rearward positions, with adult females and young occupying the more protected middle positions. As hypothesised, the Bossou chimpanzees employ a phylogenetically-old mechanism to adapt to a more recent dangerous situation. However, the positioning of dominant and bolder individuals, in particular the alpha male, changed depending on both the degree of risk and number of adult males present; dominant individuals act cooperatively with a high level of flexibility to maximise group protection. At a proximate level each individual may have preferred and recognised positions; however, it is unknown whether positioning is individual- or rank-specific. Data on progression orders of other great ape populations are required, and would help shape hypotheses about emergence of this aspect of hominoid social organisation. Acknowledgments We are grateful to the Direction ­Nationale de la Recherche ­Scientifique et Technique, the Republic of Guinea, for granting us permission to carry out this research. We would like to thank all the guides who helped ­during this ­research period. This work was ­supported by a Stirling University ­studentship, MEXT grant #16002001 and JSPS-HOPE. Supplemental data Supplemental data, with a video-clip of the Bossou chimpanzees crossing the large road, are available at http://www. current-biology.com/cgi/content/ full/16/17/R668/DC1/ References 1.Altmann, S.A. (1979). Baboon progressions: order or chaos? A study of one-dimensional group geometry. Anim. Behav. 27, 46–80. 2.DeVore, I., and Washburn, S.L. (1963). Baboon ecology and human evolution. In African ecology and Human evolution, F.C. Howell and F. Bourliere eds. (Chicago: Aldine), pp. 335–367. 3.Rhine, R.J., and Westlund, B.J. (1981). Adult male positioning in baboon progressions: order and chaos revisited. Folia. Primatol. 35, 77–116. 4.Rhine, R.J., and Tilson, R. (1987). Reactions to fear as a proximate factor in the sociospatial organization of baboon progressions. Am. J. Primatol. 13, 119–128. 5.Matsuzawa, T. (2006). Sociocognitive development in chimpanzees: A synthesis of laboratory work and fieldwork. In Cognitive Development in Chimpanzees, T. Matsuzawa, M. Tomonaga and M. Tanaka, eds. (Tokyo: Springer), pp. 3–33. 6.Sugiyama, Y. (2003). Demographic parameters and life history of chimpanzees at Bossou, Guinea. Am. J. Phys. Anthropol. 124, 154–165. 1Department of Psychology, University of Stirling, Stirling FK9 4LA, Scotland, UK. 2Primate Research Institute, Kyoto University, Inuyama-city, Aichi, 484- 8506, Japan. E-mail: k.j.hockings@stir.ac.uk Minimal plastid genome evolution in the Paulinella endosymbiont Hwan Su Yoon1, Adrian Reyes-Prieto1, Michael Melkonian2 and Debashish Bhattacharya1 It is an enduring mystery how organelles were first established in eukaryotes. A key player in this saga is the thecate amoeba Paulinella chromatophora which over 100 years ago [1] showed naturalists that once free-living cells could exist as endosymbionts [2]. This species has the honor of being the only known case of an independent primary (cyanobacterial) plastid acquisition [3,4] and is a model for understanding plastid establishment. The Paulinella plastid, often referred to as the cyanelle, retains typical cyanobacterial features such as peptidoglycan and phycobilisomes, but is considered to be a bona fide endosymbiont because it is no longer bound by a vacuolar membrane but lies free in the cytoplasm, its number is regulated, suggesting genetic integration, and it cannot be cultured outside the host [5–7]. Paulinella is, however, difficult to culture, and so it has resisted detailed molecular biological investigation. Here we took advantage of a Lambda DASH II phage library made from limited amounts of Paulinella total genomic DNA to reconstruct the evolutionary history of its recently established plastid [3]. Our data show the Paulinella plastid genome to have characteristics typical of cyanobacterial, not plastid genomes. The Paulinella library was screened with the highly conserved psbA, psbC and 16S rDNA plastid genes from the glaucophyte Glaucocystis nostochinearum. Two plastid inserts of 9.4 kb and 4.3 kb were obtained by this approach; a third, 5 kb fragment has already been described [3]. Because the P. chromatophora culture is not axenic (see [3]), we Magazine R671 isolated small subunit rDNA from the phage library to establish the identities of the different culture inhabitants. Sequence analysis revealed 20 clones from two classes of prokaryote-derived rDNA genes (see Figure S1 in the Supplemental data available on­ line with this issue). An additional PCR product was sequenced that encodes the previously reported nuclear rDNA [8] from Paulinella that has Euglyphidae homologs. The first class of prokaryotic genes, from two clones, is identical to a sequence from isolated, washed Paulinella plastids [3], and is of cyanobacterial origin. The second class is rDNA from bacterial lineages representing 18 distinct, putative culture inhabitants (Figure S1). One is a Pseudomonas-like sequence (e.g. PC9, 11 distinct clones found); one is Agrobacterium- like (PC3); two are related to Solibacter (PC1 and, PC7, two distinct clones found); and two are related to low G + C Gram-positive bacteria (e.g. PC18 and PC22, four distinct clones found). Clearly, the Paulinella culture contained a wide variety of bacteria that we have likely incompletely sampled in our PCR screen. Despite the contaminants, the identity of the cyanobacterial- like small subunit rDNA found in our work with the gene Marin et al. [3] sequenced from isolated Paulinella plastids assures us that the culture contains only a single cyanobacterial-derived rDNA sequence, derived from the plastid genome. The Paulinella plastid rDNA is most closely related to those from a clade of Synechococcus species defined by strains WH5701, BS4, and kpr27rc (Bayesian posterior probability = 1.0) within a larger clade of Prochlorococcus and Synechococcus (PS, Figure S1) cyanobacteria [3]. Alignment of the 9.4 kb plastid genome fragment with the homologous regions in PS cyanobacteria is shown in Figure 1A. This comparison reveals strong conservation of plastid gene order to Synechococcus sp. WH5701, with the level of conservation to closely related cyanobacteria approximately related to the A 0 5 10 (kb) 70 50 (%) 30 GC = 40.0 ± 7.6 % Paulinella chromatophora Plastid Synechococcus sp. WH5701 (3,044 kb) Synechococcus sp. CC9605 (2,511 kb) psb O fts H psb O cysD fts H eda psb O (9,398 nt) psb A aro C Na-T psb A nif B 308 cysD fts H eda hp (225) aro C hp hp psb A nif B 220 psb O cysD fts H eda aro C 1329 psb O psb A 1324 cysD 0 C 5 (kb) psb A gst C mp hp2 70 50 (%) 30 (4,294 nt) ABC transporter 696 1 kb 701 psb A ast E gst C mp ABC transporter hp1hp2 hp3 1 kb fts H hp GC = 39.7 ± 7.7 % Synechococcus sp. WH5701 aro C 1262 303 Synechococcus elongatus PCC6301 (2,696 kb) Paulinella chromatophora Plastid eda nifB 1267 Prochlorococcus marinus CCMP1986 (1,658 kb) B cysD hp1hp2 Gene Plastid Nucleus psb O cysD fts H eda aro C psb A nif B gst C mp ABC + + - + + + + + + + + Current Biology Figure 1. The Paulinella chromatophora plastid genome. Alignment of the (A) 9.4 kb and (B) 4.3 kb plastid genome fragments from Paulinella with homologous regions in closely related cyanobacteria. The GC-content from a sliding window analysis is shown above the plastid regions. The genes are for: photosystem II manganese-stabilizing polypeptide, PSBO; ATP-sulfurylase, CYSD; cell division protein, FTSH; KDPG and KHG aldolase, EDA; chorismate synthase, AROC; photosystem II reaction center protein D1, PSBA; elongator protein 3/MiaB/NifB, NIFB; possible sodium dependent transporter, Na-T; conserved hypothetical protein, HP, HP1, HP2, HP3; putative glutathione S-transferase, GSTC; conserved hypothetical membrane protein, MP; aspartoacylase, ASTE; ABC transporter ATP binding protein, ABC transporter. Only HP2 is homologous in Paulinella and Synechococcus WH5701. (C) Location of plastid genes in the plastid or the nucleus of other photosynthetic eukaryotes. Presence is (+) and absence is (–). phylogenetic distance. Additional evidence that the Paulinella plastid is a recent acquisition comes from the gene distribution among cyanobacteria and eukaryotes. The 9.4 kb fragment contains a number of genes that have been transferred to the nucleus in photosynthetic eukaryotes — for example, psbO is nuclear in all algae and plants (Figure 1C) — whereas only psbA and ftsH are maintained in plastid genomes. Equally significant is the elongator protein 3/miaB/nifB gene that is shared by the plastid and two Synechococcus species (Figure 1A). Unlike psbO, which is essential for photosynthesis, nifB is required for the biosynthesis of the iron–molybdenum (or iron– vanadium) cofactor used by the nitrogen-fixing enzyme nitrogenase. One would expect this gene to be lost early in plastid evolution because of the high energetic costs of nitrogen fixation, and it is absent from photosynthetic eukaryotes. The remaining 4.3 kb fragment that we found, and the 5 kb fragment reported by Marin et al. [3], encode the psbA-HP-gstC-HP ABC transporter protein (Figure 1B) and 16S rRNA-ITS1-tRNA(Ile)- tRNA (Ala)-23S rRNA, respectively. The gene order in these regions is also typical of the PS clade. These Current Biology Vol 16 No 17 R672 Figure 2. Maximum likelihood (ML) tree for four ­concatenated proteins, (FTSH, PSBA, PSBO and ­elongation factor EF-Tu, TUFA). 49 Data from all available cy57 anobacterial genome se100 100 quences are included in 90 77 this analysis. ML bootstrap 100 51 100 support values are shown Synechococcus elongatus PCC 7942 92 Synechococcus elongatus PCC 6301 on top and maximum parsi65 Trichodesmium erythraeum IMS101 98 mony bootstrap values are 98 Crocosphaera watsonii WH 8501 below. The thick branches Synechocystis sp. PCC 6803 98 62 100 Nostoc sp. PCC 7120 65 have >0.95 posterior probAnabaena variabilis ATCC 29413 ability in a Bayesian inferThermosynechococcus elongatus BP1 99 100 ence. Gloeobacter viol93 Synechococcus sp. JA-3-3Ab 99 Synechococcus sp. JA-2-3Ba aceus and the Yellowstone Gloeobacter violaceus PCC 7421 Synechococcus strains 0.01 substitution/site Current Biology (JA-3-3Ab, JA-2-3Ba) are used to root the tree. The PS clade is defined by Prochlorococcus–Synechococcus strains and is sister to the Paulinella plastid. 99 100 63 80 data demonstrate the essential ‘cyanobacterial’ nature of the Paulinella endosymbiont, and if our data are typical then the plastid genome is likely to be of cyanobacterial proportions rather than characteristic of a plastid genome. A multi-gene phylogeny using a concatenated data set of FTSH, PSBA, PSBO, and TUFA (isolated from Paulinella by PCR) confirms that the plastid originated from a member of the PS-clade (Figure 2), consistent with results of the rDNA analysis (Figure S1) and the gene order data (Figure 1). The individual protein trees are shown in Figure S2. The finding of large-scale morphological changes associated with a recent green algal engulfment in the katablepharid protist ‘Hatena’ underlines the extent to which endosymbiosis may modify organisms [9]. In the case of Paulinella, additional analyses of the plastid and the nuclear genome will help us better understand this primary endosymbiosis. A prediction is that genes required for organelle division (such as ftsZ) have been transferred to the nucleus and are now under host control. In addition, we would expect Paulinella has evolved a highly regulated transport system for directly connecting carbon metabolism of the host cell and the endosymbiont. A recent analysis shows that the main primary plastid-containing group (i.e., red, green [including plants], and glaucophyte algae) achieved PS - clade Prochlorococcus marinus MIT 9312 Prochlorococcus marinus CCMP1986 Prochlorococcus marinus NATL2A 100 Prochlorococcus marinus CCMP1375 100 83 85 Prochlorococcus marinus MIT 9211 Prochlorococcus marinus MIT 9313 100 96 100 96 Synechococcus sp. CC9902 100 100 Synechococcus sp. CC9605 90 Synechococcus sp. WH 8102 99 Synechococcus sp. WH 7805 82 90 Synechococcus sp. RS 9917 Synechococcus sp. WH 5701 Paulinella chromatophora - Plastid 100 100 this function through the co- option and retargeting of existing endomembane transporters to the plastid [10]. We suggest that the major insights into primary plastid establishment such as control of organelle division and carbon translocation [10] may come from analysis of the Paulinella nuclear genome rather than that of its recent endosymbiont. This makes Paulinella an ideal model for a complete genome sequencing project that could also include its closely related sister species P. ovalis, a heterotroph which feeds actively on cyanobacteria and other prey that have been identified in food vacuoles in its cytoplasm [7]. Comparison of these amoebal genomes will allow us to potentially identify the genetic inventions that underlie the critical transition from heterotrophy to photoautotrophy. des Süßwassers mit blaugrünen chromatophorenartigen Einschlüssen. Z. Wiss. Zool. 59, 537–544. 2.Melkonian, M., and Mollenhauer, D. (2005). Robert Lauterborn (1869–1952) and his Paulinella chromatophora. Protist 156, 253–262. 3.Marin, B., Nowack, E.C., and Melkonian, M. (2005). A plastid in the making: evidence for a second primary endosymbiosis. Protist 156, 425–432. 4.Rodriguez-Ezpeleta, N., and Philippe, H. (2006). Plastid origin: replaying the tape. Curr. Biol. 16, R53–R56. 5.Kies, L., and Kremer, B.P. (1979). Function of cyanelles in the thecamoeba Paulinella chromatophora. Naturwissenschaften 66, 578. 6.Kies, L. (1974). Elektronenmikroskopische Untersuchungen an Paulinella chromatophora Lauterborn, einer Thekamobe mit blau-grünen Endosymbionten (Cyanellen). Protoplasma 80, 69–89. 7.Johnson, P.W., Hargraves, P.E., and Sieburth, J.M. (1988). Ultrastructure and ecology of Calycomonas ovalis Wulff, 1919, (Chrysophyceae) and its redescription as a testate rhizopod, Paulinella ovalis n. comb. (Filosea: Euglyphina). J. Protozool. 35, 618–626. 8.Bhattacharya, D., Helmchen, T., and Melkonian, M. (1995). Molecular evolutionary analyses of nuclear-encoded small subunit ribosomal RNA identify an independent rhizopod lineage containing the Euglyphina and the Chlorarachniophyta. J. Eukaryot. Microbiol. 42, 65–69. 9.Okamoto, N., and Inouye, I. (2005). A secondary symbiosis in progress? Science 310, 287. 10.Weber, A.P., Linka, M., and Bhattacharya, D. (2006). Single, ancient origin of a plastid metabolite translocator family in Plantae from an endomembrane-derived ancestor. Eukaryot. Cell 5, 609–612. 1Department of Biological Sciences and Roy J. Carver Center for Comparative Genomics, University of Iowa, 446 Biology Building, Iowa City, Iowa 52242-1324, US. 2Botanisches Institut, Lehrstuhl I, Universität zu Köln, Gyrhofstr. 15, 50931 Köln, Germany. E-mail: Debashi-Bhattacharya@uiowa. edu; Michael.Melkonian@uni-koeln.de The editors of Current Biology Supplemental data Supplemental data are available at http://www.current-biology.com/cgi/ content/full/16/17/R670/DC1/ welcome correspondence on any article in the journal, but reserve the right to reduce the length of any letter to be Acknowledgments This research was supported by grants from the NSF and NASA awarded to D.B. (EF 04-31117, NNG04GM17G). We acknowledge technical help from L. Pingel and M. Wu (University of Iowa). published. All Correspondence References should be sent by e-mail to 1.Lauterborn, R. (1895). Protozoenstudien II. Paulinella chromatophora nov. gen., nov. spec., ein beschalter Rhizopode cbiol@current-biology.com containing data or scientific argument will be refereed. Queries about articles for consideration in this format Supplemental Data: Minimal plastid genome evolution in the Paulinella endosymbiont Hwan Su Yoon, Adrian Reyes-Prieto, Michael Melkonian and Debashish Bhattacharya Supplemental Experimental Procedures Library Construction Total genomic DNA was isolated from pooled cultures of Paulinella chromatophora as described in Bhattacharya et al. [S1] and digested with EcoRI. Due to the small amount of total DNA that was recovered, we cloned directly the inserts without size-fractionation. In addition, the Paulinella cells grew with bacteria therefore the majority of the DNA was prokaryotic. About 100 ng of the digested DNA was used to prepare a phage library using EcoRI-digested, dephosphorylated vector LambdaDash II (9 – 23 kb cloning capacity [Stratagene, La Jolla, CA, USA]). Ligation and packaging of the vector and DNA were done according to the manufacturer’s instructions. The library was amplified (ca. 106,000 original PFU) and stored in SM buffer (10 mM NaCl, 10 mM MgCl2, 50 mM Tris-HCl [pH 7.5], 2% w/v gelatin) with 5% chloroform. The genomic bank of Paulinella was screened with a probe cocktail containing sequences of the highly conserved psbA, psbC, and 16S rDNA plastid genes from the glaucophyte Glaucocystis nostochinearum using standard methods (e.g., [ S2]). The Glaucocystis DNA fragments were isolated using PCR (as in [S3-S5]). Filters were washed two times in 2X SSC, 0.1% SDS at 40°C prior to autoradiography. Secondary and tertiary screens were used to isolate single plaques containing the target genes. The phage inserts (9.4 kb and 4.3 kb) were amplified using long-range PCR (Qiagen, Valencia, CA, USA) that were directly sequenced using primer walking and the BigDyeTM Terminator Cycle Sequencing Kit (PE-Applied Biosystems, Norwalk, CT, USA), and an ABI-3700 at the Roy J. Carver Center for Comparative Genomics at the University of Iowa. The genes encoded by the two inserts, tufA, and small subunit rDNA have been released to GenBank under the accession numbers, DQ789029 – DQ789040. PCR Isolation of Small Subunit rDNA To identify the inhabitants in the Paulinella culture, we isolated prokaryote-derived small subunit rDNA sequences using specific primers that are complementary to the 5’- and 3’-termini of these genes (SG1, SG2; see [S3, S6]). For the nuclear rDNA, we used the primers described in Bhattacharya et al. [S1]. PCR products were cleaned using the QIAquick gel Purification kit (Qiagen) and directly sequenced (nuclear rDNA) or cloned into the pGEM-T vector (Promega). A total of 20 clones encoding prokaryote-derived rDNA were picked and the inserts were sequenced as described above. Plasmid (T3, T7), external PCR, and internal genic (e.g., p651F, p16SR) primers were used to sequence the inserts [S1, S3, S6]. 1 Phylogenomic Analysis We used a phylogenomic pipeline (as in [S7, S8]) with 6 genes (psbO, cysD, ftsH, eda, aroC, psbA) from the 9.4 kb and 4 genes (psbA, gstC, hypothetical membrane protein [mp], ABC transporter) from the 4.3 kb Paulinella cyanelle fragment and a tufA fragment that was isolated from the phage library using PCR as a query against a local data base of 23 complete or nearly-complete cyanobacterial genomes and complete genome data from 21 other bacteria, 10 plastids, and 6 eukaryotes. All significant hits (BLAST e-value < 10-10) to each of the Paulinella sequences were identified and stored. The resulting alignments were manually refined and submitted to protein phylogenetic analyses. Phylogenetic Analysis The phylogenies of 7 individual Paulinella proteins and of a concatenated data set of 4 highly conserved sequences (psbO, ftsH, psbA, tufA) were reconstructed under maximum likelihood (ML) using proml in the PHYLIP V3.6b program package [S9]. The trees were inferred with the JTT + Γ evolutionary model and global rearrangements with 4 random addition replicates. The alpha values for the gamma distribution for the different data sets were calculated using PHYML V2.4.3 [S10] and the same evolutionary model. To assess the stability of monophyletic groups in the ML trees, we calculated bootstrap support values using PROML (100 replicates). For the 4-protein data set we also used unweighted maximum parsimony (MP) using PAUP*V4.0b10 [S11] and calculated Bayesian posterior probabilities (BPP) using MrBayes (V3.0b4, [S12]. In the Bayesian inference of the amino acid data, we used the WAG + Γ + I model with Metropolis-coupled Markov chain Monte Carlo from a random starting tree. These analyses were run for 1,000,000 generations with trees sampled each 200 cycles. Four chains were run simultaneously of which 3 were heated and one was cold, with the initial 20,000 cycles (200 trees) being discarded as the “burn in”. A consensus tree was made with the remaining 1,800 phylogenies to determine the posterior probabilities at the different nodes. In the MP analyses, 1000 bootstrap replicates were analyzed with ten heuristic searches with random-additionsequence starting trees and tree bisection-reconnection (TBR) branch rearrangements. For the rDNA tree of the different genes isolated from the Paulinella library, we inferred a PHYML tree using the GTR + Γ + I model and tested support for clades in this phylogeny with PHYML analysis of 100 bootstrap replicates. In addition, we did MP bootstrap and Bayesian analyses with the rDNA data as described above. GC-Content, Gene Order, and Gene Presence/Absence Analyses We used the SWAAP (V1.0.2 http://www.bacteriamuseum.org/SWAAP/SwaapPage.htm) computer program to calculate GC-content across the Paulinella cyanelle fragments using a sliding window approach. Gene order was compared between the Paulinella 9.4 kb and 4.3 kb fragments with homologous regions in sequenced genomes of closely related cyanobacteria using the NCBI microbial genome database (http://www.ncbi.nlm.nih.gov/genomes/lproks.cgi). The results of this analysis were 2 manually prepared for presentation (see Fig. 1 in text). Gene presence/absence in plastid versus nuclear genomes for the coding regions in both cyanelle fragments was done as follows. First, phylogenomics using the local database described above was used to identify all orthologs in the organellar and nuclear genomes to identify their cellular location. To verify these results, we then did a BLAST search against the NCBI non-redundant database to search for all available orthologs. The results of these two analyses were used to infer the putative cellular location of the Paulinella cyanelle genes across photosynthetic eukaryotes. Supplemental References S1. Bhattacharya, D., Helmchen, T., and Melkonian, M. (1995). Molecular evolutionary analyses of nuclear-encoded small subunit ribosomal RNA identify an independent rhizopod lineage containing the Euglyphina and the Chlorarachniophyta. J. Eukaryot. Microbiol. 42, 65-69. S2. Bhattacharya, D., and Weber, K. (1997). The actin gene of the glaucocystophyte Cyanophora paradoxa: analysis of the coding region and introns, and an actin phylogeny of eukaryotes. Curr. Genet. 31, 439-446. S3. Yoon, H.S., Hackett, J.D., Pinto, G., and Bhattacharya, D. (2002). The single, ancient origin of chromist plastids. Proc. Natl. Acad. Sci. USA 99, 15507-15512. S4. Yoon, H.S., Hackett, J.D., Ciniglia, C., Pinto, G., and Bhattacharya, D. (2004). A molecular timeline for the origin of photosynthetic eukaryotes. Mol. Biol. Evol. 21, 809-818. S5. Yoon, H.S., Hackett, J.D., and Bhattacharya, D. (2002). A single origin of the peridinin- and fucoxanthin-containing plastids in dinoflagellates through tertiary endosymbiosis. Proc. Natl. Acad. Sci. USA 99, 11724-11729. S6. Oliveira, M.C., and Bhattacharya, D. (2000). Phylogeny of the Bangiophycidae (Rhodophyta) and the secondary endosymbiotic origin of algal plastids. Am. J. Bot. 87, 482-492. S7. Li, S., Nosenko, T., Hackett, J.D., and Bhattacharya, D. (2006). Phylogenomic analysis identifies red algal genes of endosymbiotic origin in the chromalveolates. Mol. Biol. Evol. 23, 663-674. S8. Reyes-Prieto, A., Yoon, H.S., and Bhattacharya, D. (2006). Phylogenomics and its growing impact on algal phylogeny and evolution. Algae 21, 1-10. S9. Felsenstein, J. (2002). PHYLIP (Phylogeny Inference Package) 3.6, Department of Genetics, University of Washington: Seattle, WA. S10. Guindon, S., and Gascuel, O. (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696-704. S11. Swofford, D.L. (2004). PAUP*: Phylogenetic Analysis Using Parsimony (* and Other Methods) 4.0b10, Sinauer: Sunderland, MA. S12. Huelsenbeck, J.P., and Ronquist, F. (2001). MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754-755. 3 Figure S1. Small subunit rDNA tree of plastid and nuclear encoded genes showing the origins of the 20 prokaryote-derived and the nuclear sequence found in the Paulinella chromatophora culture. This is a maximum likelihood (ML) phylogeny with ML bootstraps shown at the branches on the left of the 5 slash marks and maximum parsimony (MP) bootstrap values on the right. The thick branches have >95% posterior probability in a Bayesian inference. The branch connecting the nuclear (18S) and plastid/bacterial (16S) small subunit rDNAs has been truncated to focus attention on the branching order within the two clades. 6 Figure S2. ML trees of all proteins identified on the 9.4 kb Paulinella cyanelle fragment and TUFA. Data from all sequenced (at the time of writing) cyanobacterial genomes are included in this analysis. ML bootstrap support values are shown above the branches. Gloeobacter violaceus and/or the Yellowstone Synechococcus strains (JA-3-3Ab, JA-2-3Ba) are used to root the tree. 7