Advanced Placement Physics “B”
advertisement

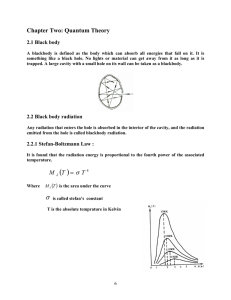
Advanced Placement Physics “B” Mr. Mirro Type: Date: ______________ Day #135 Period #201 Single – Theory Objective: Teaching notes: Animations http://webphysics.davidson.edu/alumni/MiLee/java/bb_mjl.htm http://www.lon-capa.org/~mmp/applist/blackbody/black.htm http://highered.mcgraw-hill.com/sites/0072482621/information_center_view0/interactives.html# Homework: Assignment (102) Read (29.1-29.2) Advanced Placement Physics “B” Mr. Mirro Date: ___________ Introduction to Quantum Theory THE BLACKBODY RADIATION PROBLEM As the nineteenth century came to a close, physicists in general were very confident about their ability to describe the physical world around them. Using Newton's Laws as a foundation, problems that had befuddled scientists for centuries were being explained almost nonstop. The Industrial Revolution, again fueled by Newton's picture of the Universe, had provided further proof that the physical world was explainable using experimentation and mathematics. • In classical thermodynamics, the equipartition of energy theorem showed that interacting systems, on average, must exchange similar amounts of energy with each other. • With the addition of Maxwell's equations of light, Integral form Differential form I. Gauss' law for electricity II. Gauss' law for magnetism III. Faraday's law of induction IV. Ampere's law V. Maxwell’s additional term ∫ Bdl=μ 0 i + μ0 ™0 d ΦE dt ∇ x B = μ0 j + μ0 ™0 ∠E ∠t it seemed as if a physicist could solve ANY problem put forth. However … in 1859, the "blackbody radiation" problem was first raised by Gustav Kirchoff. • • The term "blackbody" indicates an object that absorbs all energy that falls upon it, but … It can also mean an object that emits energy 100% efficiently. A black body radiator is a theoretical model for an object that absorbs ALL of the radiation that hits it. Therefore, a “perfect” black body is said to reflect NO radiation and thus “appears” perfectly BLACK. Thus, if the black body is hot, these properties make it an ideal source of thermal radiation. If a perfect black body at a certain temperature is surrounded by other objects in thermal equilibrium at the same temperature, it will on average emit exactly as much as it absorbs, at every wavelength. Actually, in practice no material has been found to absorb ALL incoming radiation. However, Carbon, in its graphite form, absorbs all - but about 3% of the incident radiation. Carbon also happens to be an exceptionally good emitter of radiation as well. A good model of a blackbody radiator, ironically, is the Sun. Kirchoff noticed that the Sun emits all possible wavelengths, even ones in the infrared and ultraviolet regions of the electromagnetic spectrum. We know now that the sun actually emits waves from every region of the EM spectrum, but Kirchoff could not have measured this. The Sun emits most of its energy in the form of yellow light, while a relatively small fraction of the energy is located in the ultraviolet and infrared regions. Further experiments proved that the peak color would change for different temperatures. The graphs below show how this peak color changes as the temperature changes: Undoubtedly you have noticed that the hottest part of a flame, near the wick of a candle for instance, is blue while a dying ember is red. The same rules apply for a blackbody, however according to Maxwell's equations, a blackbody cannot have a peak color - it must radiate EVERY wavelength equally. This would mean that the Sun is radiating so many ultraviolet waves to match the visible ones that it would require an infinite amount of energy for the Sun to shine ! THIS ABSURD PROPOSITION IS ACTUALLY CALLED "THE ULTRAVIOLET CATASTROPHE" BECAUSE, FOR THE FIRST TIME SINCE 1687, THE LAWS OF PHYSICS DID NOT WORK ! The blackbody radiation problem went largely unsolved until 1900 when Max Planck, in a fit of frustration, proposed the following "ridiculous" theory: “MAYBE A STAR, OR ANY BLACKBODY, CAN ONLY EMIT CERTAIN WAVELENGTHS. Working on this hypothesis, Planck discovered that this is exactly the case - the energy of a heated body is NOT continuous spectrum, as Maxwell predicted, but rather it is made up of "energy chunks," which Planck named "quanta." Hence, the branch of physics known as "quantum mechanics" was born. The difference between Maxwell's and Planck's ideas on energy can be best described with an analogy: Maxwell - imagined energy as a fully stocked bank or cash register. ie. A customer could ask for and receive any amount of money they desired, be it one penny, a million dollars or any amount in between. Planck - viewed energy more like an ATM that is programmed to dispense money in multiples of some base amount like $20. ie. As long as the customer asks for an amount that is a multiple of twenty dollars, the machine will comply. However, a request of $8.76 will be denied. This does not mean such an amount does not exist, it just means that the programming will not allow this amount to be dispensed. In the case of a blackbody, the "programming" tells the star exactly what types and how much of each wave can be emitted. • • The Sun emits mostly yellow light because that is what it is programmed to do. Likewise… The same-programming limits the amount of ultraviolet and infrared that the Sun can emit. Instead of “programming” - we say that all energy is "quantized.” So how much energy does a quanta have? We know from the electromagnetic spectrum that the higher the wave's frequency (f), the higher the energy (E). Therefore, the equation to find the energy of an electromagnetic wave can be expressed as: EPHOTON = hf Where, "h" is known as Planck's constant and has a value of h = 6.63 x 10-34 Joule-sec (or J / Hz) “or” h = 4.14 x 10-15 eV-sec (or eV / Hz) AP Physics “B” Mr. Mirro Date: ________ Introduction to Quantum Theory (∅) Ex 1: Plank’s constant (h = 6.63x10-34 J sec) is incredibly small, so small that some physicists believe that this is the smallest measurement possible. Its “small size” may explain why it was not discovered until the twentieth century. This also means that the energy for a solitary quanta, even for a high frequency gamma rays of f =1x1024 Hz is still very small. Compute the energy of that each gamma ray posses. Ex 2: Physicists dealing with quantum energies find that the Joule (J) is too large to measure energy with. Instead, an energy unit called the electron-volt (eV) is used. An electron-volt is the amount of energy necessary to move one electron across a one volt of potential difference. Therefore, one electron-volt is equal to 1.6 x 10-19 Joules. Based upon your answer from example #1, convert the energy that the gamma ray would have into electron-volts. Ex 3: a) Calculate the amount of energy, in electron-volts, carried by a 2 x 1010 Hz microwave signal using Planck’s constant h = 4.14 x 10-15 eV-sec. b) Convert the energy of the microwave into joules. Ex 4: What is the wavelength of a wave that carries 0.4 eV of energy?