Semi-conductors
advertisement
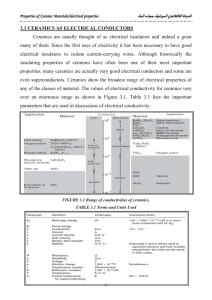
Semi-conductors Properties of Solids • Electrical (Conducting) properties of solids: – Conductors – Insulators – Semi-Conductors • The electrical conductivity at room temperature is quite different for each of these three kinds of solids – Metals and alloys have the highest conductivities – followed by semiconductors – and then by insulators (Super)conductor / Semiconductor Superconductor Wave functions overlap • When two atoms approach each other, their wave functions overlap • Bonding / Antibonding depends on relative sign of the two wave functions Bonding Antibonding The Hydrogen Molecule Many Atoms 2 hydrogen atoms • When many atoms approach each others, more energy states are formed Create bands separated by energy gaps 11 hydrogen atoms Band Theory of Solids • The Kronig-Penney Model (1931) – Interaction between the electrons and the lattice of atoms Lattice Coulomb field Simplified model (Kronig/Penney) Kronig-Penney Model (I) • Electron: – essentially free between 0<x<a (and in the similar region along the lattice) – Has to tunnel through the barrier regions Kronig-Penney Model (II) • Solutions: – “Free electron” region: • With: – Barrier – tunneling: • With: • Boundary Conditions: K, another wave number Allowed / Forbidden Regions FORBIDDEN REGIONS What’s what ? Band Theory Electron No ! • Lower band: valence band • Upper band: conduction band (Intrinsic) Semiconductors Copyright HyperPhysics • Energy gap smaller than 1 eV or so. • Fermi level ~ in the middle of the gap • Thermal excitation electrons can jump to the conduction band – Two types of charge “carriers”: • Electrons in the conduction band • Holes (vacancies) in the valence band Temperature and Resistivity • The increased number of electrons in excited states explains the temperature dependence of the resistivity of semiconductors. • A useful empirical expression developed by Clement and Quinnell for the temperature variation of standard carbon resistors is given by where A, B, and K are constants. Semi-conductors can be used as thermometers (Thermoelectric effect. See: Thermocouples p399) Impurity Semiconductors electron • It is possible to improve very significantly the conduction properties by “doping” the semiconductor. – N-type: dopant adds electrons levels close to the conduction band extra electrons can jump in the conduction band from these donor levels – P-type: dopant adds vacancy levels close to the valence band electrons from the valence band can jump in the acceptor levels leaving holes/vacancies in the valence band Example: Silicon • Silicon: 3s23p2 • Arsenic: 4s24p3 One more electron, donor level • Indium: 5s25p1 One less electron, acceptor level Applications: Diodes • pn junction Inverse Bias No Bias (p) (n) One application: Wheatstone Bridge – a.k.a Bridge Rectifier Forward Bias Applications: Photodiodes / Photovoltaic Cells • LED (Light Emitting Diode): • Solar Cell: Applications: Transistors Voltage and Current Amplification Applications: Integrated Circuits Moore’s law, showing the progress in computing power over a 30-year span, illustrated here with Intel chip names. The Pentium 4 contains over 50 million transistors. Courtesy of Intel Corporation. Graph from http://www.intel.com/research/silicon/mooreslaw.htm.