The electronic structure of solids
advertisement

The electronic structure of solids
We need a picture of the electronic structure of
solid that we can use to explain experimental
observations and make predictions
Why is diamond an insulator ?
Why is sodium a metal ?
Why does the conductivity of silicon increase
when you heat it ?
Charge transport in solids
The conductivity of a material is determined by
three factors:
– the charge on the charge carriers
– the number of charge carriers
– the mobility of the charge carriers
σ=neµ
Page 1
Conductivity of common materials
Very
large variation in conductivity
A simple model for metals
Consider a metal such as Na, Mg or Al to be
essentially a box in which the valence electrons of
the metal are confined.
The potential within the box is taken to be
uniform and much lower than that in the
surrounding medium.
Treat quantum mechanically as a particle in a box
Page 2
The Free Electron Theory
You can get solutions of the form
– ψn(r) = A sin(πnxx/L) sin(πnyy/L) sin(πnzz/L)
– these solutions are standing waves
Adjust the boundary conditions to get traveling
waves
– ψk(r) = exp (i k.r), k is the wavevector
– εk = (h2/2m) (kx2 + ky2 + kz2)
– k can not take all values but in many electron systems it
is almost continuous
k, the wavevector
k is related to the momentum of the electrons in
the orbital
– p = hk
k is related to the wavelength of the electron wave
– |k| = 2 π / λ
Page 3
Solutions to a three dimensional
particle in a box problem
E = (n2x + n2y + n2z)h2 / (8ma2)
For a large box the energy levels are going to
be close together
Consider energy levels as forming a
continuous band
How many energy levels do we have with
energy less than some critical value ?
Number of electrons below Emax
We can have two electrons per unique
combination of nx, ny and nz
Set R2 = n2x + n2y + n2z
N = 2 (1/8) (4/3)π
π R3max
= (8π
π/3)(2mEmax/h2)3/2 a3
Page 4
N(E) - Density of States (DOS)
N(E) = 4π(2m/h2)3/2 E1/2
At temperatures above 0 K some higher energy states are
occupied, f(E) = {1+ exp[E-EF)/kT]}-1
X-ray emission spectra for
sodium and aluminum
Spectra
strongly resemble simple parabolic density of
states predicted by free electron model
Na
Al
Page 5
Temperature dependence of
electron distribution
At
temperatures above 0K some electron promoted to
states with higher k.
Electron distribution described
by Fermi-Dirac distribution
function
f (E ) =
1
1 + exp[(E − EF ) / KT ]
The wavevector k
Classically the kinetic energy of an electron is
given by E = p2 / 2m
The free electron model gives the energy of an
electron as, E = (n2x + n2y + n2z)h2 / (8ma2)
The momentum p is usually expressed as kh so E
= (k2x + k2y + k2z) h2 /2m
Page 6
Electrical conductivity
In the absence of an electric field states
corresponding to k and -k are equally likely
to be populated so there is no overall
movement of charge
In the presence of an electric field states
with the same |k| but differing k do not
necessarily have the same energy. This can
lead to charge transport.
Electrical conductivity
Page 7
Limitations of the free electron model
Predicts all materials will be metals !
The tight binding approximation
Consider a solid to
be a large molecule
and apply molecular
orbital theory
Page 8
MOs for evenly spaced H atoms
Solids have MOs that are so close in energy
they form continuous bands
H
H2
H4
H9
Hn
Chains of CH units
Consider polyene chains
Evenly spaced
(CH)H2
(CH)2H2
(CH)4H2
(CH)8H2
(CH)nH2
Bond alternation
Page 9
Electronic structure of NaCl
The band gap
The
occurrence of
groups or “bands”
of orbitals with
energy gaps in
between them is
common
Page 10
The origin of band gaps
The chemists view
– atoms in solids have orbitals that overlap to produce
“large molecular orbitals”
– These “molecular orbitals” do not occur at all energies
The physicists view
– we need to modify the theory to take into account the
periodicity of the structure
– electron waves can be diffracted by a regular array on
ions in a solid
Variation of band width and
overlap with interatomic distance
Pushing
atoms closer together increases orbital
overlap and increases band widths
Calculated for Na
using TBA
Page 11
Variation of conductivity with pressure
As
pressure effects interatomic distances and band
widths it can have a profound influence on electronic
conductivity
What are the coefficients for the
orbitals in the bands?
Consider a chain of atoms
Use LCAO, Ψ(x) = Σ cnψn(x)
The periodicity of the chain limits the
possible solutions for cn
cn = exp(ikna)
Page 12
Bloch functions for 1D chain
Conductivity of solids
This approach to the electronic structure of
solids naturally introduces electronic states
(orbitals) with characteristic momentum p=hk
Electrical conductivity can again be related to
differing numbers of electrons in states with
+k and -k
Conductivity is limited by lattice vibrations
(phonons) in metals
Page 13
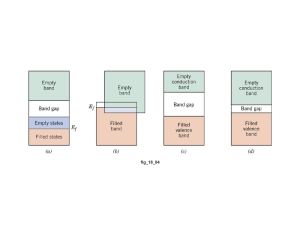
Bands in metals, semiconductors
and insulators
E
E
Metal
E
Intrinsic
semiconductor
Insulator
Insulators
All bands are fully occupied or empty
making it impossible for more electrons to
be in states with +k rather than -k
Page 14
The band structure of group IV elements
Intrinsic and extrinsic semiconductors
In
an intrinsic semiconductor the conduction band is
populated by thermal excitation of electrons from the
valence band
In an extrinsic semiconductor doping is used to
produce partially occupied bands
Page 15
Band gaps for Group IV Elements
Band gaps for inorganic compounds
Page 16
Doping semiconductors
The addition of very small amounts of
dopant can dramatically influence
properties
– P, As added to silicon gives n - type material
– B, Al, Ga gives p - type material
The conductivity of doped semiconductors
varies less with temperature
Extrinsic semiconductors
Doping can be used to increase the
conductivity of a semiconductor
conduction band
E
E
valence band
p doping
n doping
Page 17
Temperature dependence of
electron distribution
Temperature dependence of conductivity
The conductivity of a metal decreases with increasing
temperature
– mobile electrons are scattered by lattice vibrations
The conductivity of a semiconductor increases with increasing
temperature as more charge carriers become available
Page 18
Doped graphites
Graphite is a semimetal
– doping with bromine or potassium improves its
conductivity
Br2
E
E
K
E
TiO2 , VO2 and TiS2
TiO2 is an insulator as the d-bands are empty
VO2 at higher temps is metallic as the d-band is partly filled
TiS2 is a metal as S 3p and Ti 3d bands overlap
Page 19
LixV2O5
V2O5 has an empty d band and a layered
structure
Intercalation of Li into the material dopes
the V2O5
– puts electrons in to the empty d band
This improves the solids conductivity
VO2
VO2 has a rutile like structure
– chains of edge sharing VO6 octahedra
– V(IV) has d1 electron configuration
– at low temperatures it displays localized metalmetal bonds and is a semiconductor
– at high temperatures the structural distortion
disappears and it is a metal
Page 20
Phase transitions in VO2
metal d band
splits
E
oxygen band
High T
Low T
Polyacetylene
Polyacetylene is a semiconductor because it
displays bond alternation
– without bond alternation it would be a metal
It can be doped to make it conducting
– use oxidizing agents, AsF5, I2 etc. to remove
electrons from the valence band
Page 21
K2[Pt(CN)4]Br0.3.3H2O
In KCP the Pt dz2 orbitals are in an evenly
spaced chain (at room temp) forming a single
band
E
Metal
Insulator
K2[Pt(CN)4]
Bromine
doped
Structure of K2[Pt(CN)4]Br0.3.3H2O
1D
chain compound with overlapping dz2 orbitals
Page 22
Peierls distortion
On cooling below 150K the conductivity of
KCP drops rapidly
– this is associated with a lattice distortion
E
Page 23