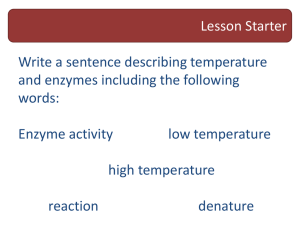
Enzyme Activity Guided Inquiry Lab Turnip Peroxidase Introduction
advertisement

Publication No. 11137 Enzyme Activity Guided Inquiry Lab Turnip Peroxidase Introduction Peroxidase enzymes are widely distributed in plants and animals, including bacteria, to protect cells against the effects of oxidative stress and cell damage due to hydrogen peroxide. Peroxidases are easily extracted from turnips and other root vegetables and provide a model enzyme for studying enzyme activity—how the rate of an enzyme-catalyzed reaction depends on biotic and abiotic factors. Enzyme activity studies reflect enzyme structure and function and provide the foundation for understanding the mechanism or theory of enzyme action. Background The term peroxidase refers to both a class of oxidoreductase enzymes and to specific enzymes within that class. As a general class of enzymes, peroxidases catalyze the oxidation−reduction decomposition reaction of hydrogen peroxide. There are two general types of peroxidases—catalase and peroxidase. Catalase catalyzes the disproportionation reaction of hydrogen peroxide to water and oxygen gas (Equation 1). In reactions mediated by catalase, hydrogen peroxide substrate molecules act as both oxidizing agent (electron acceptor) and reducing agent (electron donor). In contrast, peroxidase acts in the presence of other naturally occurring organic reducing agents, such as ascorbic acid and glutathione, to catalyze the decomposition of hydrogen peroxide. Organic reducing agents, abbreviated AH2, transfer hydrogen atoms and electrons to hydrogen peroxide, resulting in the formation of water and oxidized organic substrates such as A2 in Equation 2. Catalase-catalyzed reaction Peroxidase-catalyzed reaction 2H2O2 → 2H2O + O2 Equation 1 2H2O2 + 2AH2 → 4H2O + A2 Equation 2 The differences in the two equations shown above provide a basis for studying the enzyme activity of turnip peroxidase in this guided-inquiry laboratory investigation. Many endogenous organic compounds may be used as reducing agents in Equation 2. One of the most common and convenient reducing agents for this purpose is guaiacol, a colorless compound having the formula C7H8O2. Oxidation of guaiacol according to Equation 2 converts it to a dark orange compound called tetraguaiacol. The rate of the reaction may be followed by measuring the absorbance or color intensity of the orange product as a function of time. Materials Buffer capsules, pH 3−8, 100 mL each Erlenmeyer flask, 500-mL Distilled or deionized water Filter paper and funnel Guaiacol, C7H8O2, 1 mL Hot plate Hydrogen peroxide, H202, 3%, 3 mL Knife, paring Isopropyl alcohol, (CH3)2CHOH 70%, 100 mL pH paper, narrow range Phosphate buffer, pH 7, 300 mL, NaH2PO4 and Na2 HPO4, 0.05 M Pipets, serological, 2- and 5-mL Turnip (root/tuber) Spectrophotometer Ice and water baths Test tubes, 13 × 100 mm, 6, and rack BlenderThermometer Timer BIO-FAX. . .makes science teaching easier. 113712 032712 Safety Precautions Guaiacol is toxic by ingestion. It has an aromatic, creosote-like odor and may be irritating to the nose and throat. Isopropyl rubbing alcohol (70%) is a flammable liquid. Keep away from heat, flames, and other sources of ignition. Dilute hydrogen peroxide solution (3%) may be irritating to the eyes and skin. Exercise care when using a knife to peel and cut the turnip. Avoid contact of all chemicals with eyes and skin. Wear chemical splash goggles, chemical-resistant gloves, and a chemical-resistant apron. Please review current Material Safety Data Sheets for additional safety, handling, and disposal information. Preparation 1. Extraction Buffer: Prepare 500 mL pH 7 phosphate buffer by mixing equal volumes, 150 mL each, of 0.1 M sodium phosphate monobasic and sodium phosphate dibasic solutions. Verify pH using narrow-range pH paper; it should be between 6.8 and 7.2. 2. Reaction Buffers: Dissolve one each pH 3−8 buffer capsules in 100 mL distilled or deionized water according to instructions. 3. Guaiacol Solution: Dissolve 0.2 mL guaiacol in 100 mL of isopropyl rubbing alcohol. Store in a dark bottle away from heat and light. 4. Hydrogen Peroxide: Dilute 3 mL of 3% hydrogen peroxide to a final volume of 500 mL using distilled or deionized water. Store in a dark bottle protected from heat and light. Enzyme Extraction Peel and cut a turnip root into small cubes, about 1 cm on each side. Measure approximately 2 g (about 2 pieces) in a weighing dish and add to 500 mL of pH 7 phosphate extraction buffer in a blender. Pulse the turnip root in 1−3 minute bursts three times, with 2-minutes rest between pulses, to homogenize and extract the enzymes. Filter the enzyme extract through filter paper and store the extract over ice or in the refrigerator. Baseline Activity—Peroxidase-Catalyzed Oxidation Decomposition of Hydrogen Peroxide Read the entire procedure before beginning. Pay special attention to the requirements for mixing the tubes and timing the reaction. Accurate timing is crucial for rate studies. 1.Turn on the spectrophotomer, adjust the wavelength setting to 500 nm, and allow the instrument to warm up for 15–20 minutes. 2. Prepare separate 13 × 100 mm test tubes containing substrates (tube S) and enzyme (tube E) in pH 5 buffer as shown below. The presence of pH 7 extraction buffer in tube E makes it possible to vary the enzyme concentration while maintaining the overall buffer composition constant. The concentrations of the diluted hydrogen peroxide and guaiacol solution are described in the Preparation section. Tube S: 2 mL pH 5 buffer Tube E: 2 mL pH 5 buffer 2 mL dilute H2O2 1.5 mL pH 7 phosphate extraction buffer 1 mL dilute guaiacol 0.5 mL enzyme extract 3. Prepare a “blank” by combining 4 mL pH 5 buffer, 2 mL dilute H2O2, 1 mL dilute guaiacol, and 2 mL pH 7 phosphate buffer in a 13 × 100 mm test tube. 4. Zero the spectrophotometer (zero absorbance, 100% transmittance) at 500 nm using the blank solution. 5. When ready to begin a kinetics run, carefully pour the contents of tube S into tube E and immediately start timing. Carefully pour the combined contents back into tube S, wipe the outside of the tube with lab tissue, and place the test tube in the spectrophotometer cell holder. 6. Measure and record the absorbance as a function of time every 20 seconds. Ideally, the elapsed time between mixing the tubes and recording the first absorbance measurement should be no more than 20−40 seconds! –2– © 2012 Flinn Scientific, Inc. All Rights Reserved. 11137 Enzyme Activity—Opportunities for Inquiry Vary the enzyme and substrate concentrations and investigate the effects of pH, temperature, and possible inhibitors on the rate of the peroxidase-catalyzed decomposition of hydrogen peroxide. Analyze and graph the results and explain in terms of the mechanism of enzyme action and structure−function relationships involving biological protein molecules. Precise volume measurements are essential for meaningful results and discussion. AP Biology Curriculum Framework (2012) Essential Knowledge 2.D.1. All biological systems from cells and organisms to populations, communities and ecosystems are affected by complex biotic and abiotic interactions involving exchange of matter and free energy. Essential Knowledge 4.A.1. The subcomponents of biological molecules and their sequence determine the properties of that molecule. Essential Knowledge 4.B.1. Interactions between molecules affect their structure and function. Sample Results Effect of Enzyme Concentra9on 0.80 0.70 0.40 0.60 [E] = 2x [E] = x [E] = 0.5 x 0.50 0.40 y = 0.0008x R² = 0.9984 0.30 0.20 y = 0.0004x R² = 0.9698 0.10 0.00 y = 0.0008x R² = 0.9984 0.35 Absorbance at 500 nm Absorbance at 500 nm Effect of Substrate Concentra9on y = 0.0016x R² = 0.9957 0.30 0.20 100 200 300 400 500 0.15 600 0 100 200 300 400 500 600 Time (sec) Effect of Substrate Concentra.on on Reac.on Rate y = 0.0008x R² = 1 1.00 1.50 Rate, Delta Abs/sec Rate, Delta Abs/sec [S] = 0.25x 0.05 Effect of Enzyme Concentra.on on Reac.on Rate 0.50 [S] = 0.5x y = 0.0005x R² = 0.959 Time (sec) 0.0018 0.0016 0.0014 0.0012 0.0010 0.0008 0.0006 0.0004 0.0002 0.0000 0.00 [S] = 2x 0.10 0.00 0 y = 0.0007x R² = 0.9736 0.25 2.00 2.50 Rela.ve Enzyme Concentra.on 0.0009 0.0008 0.0007 0.0006 0.0005 0.0004 0.0003 0.0002 0.0001 0 0.0 0.5 1.0 1.5 2.0 2.5 Rela.ve Substrate Concentra.on –3– © 2012 Flinn Scientific, Inc. All Rights Reserved. 11137 0.0025 1.6 0.0020 1.4 Rate, Delta Abs/sec Rate, Delta Abs/sec Effect of Temperature on Enzyme Ac:vity Effect of pH on Enzyme Ac8vity 1.8 1.2 1.0 0.8 0.6 0.4 0.2 0.0 pH 3 pH 4 pH 5 pH 6 pH 7 0.0015 0.0010 0.0005 0.0000 pH 8 9 deg C 23 deg C 35 deg C 45 deg C 50 deg C Temperature Materials for Enzyme Activity Guided Inquiry Lab are available from Flinn Scientific, Inc. Catalog No. B0227 B0099 G0054 H0009 I0021 AP4869 Description Buffer Capsules, pH 2–12 Buffer Solution Concentrated, pH 7, 500 mL Guaiacol, 20 mL Hydrogen Peroxide, 3%, 473 mL Isopropyl Alcohol, 70%, 500 mL Blender, Single-Speed Consult your Flinn Scientific Catalog/Reference Manual for current prices. –4– © 2012 Flinn Scientific, Inc. All Rights Reserved. 11137