Chemistry of Benzene Reactions of Benzene
advertisement
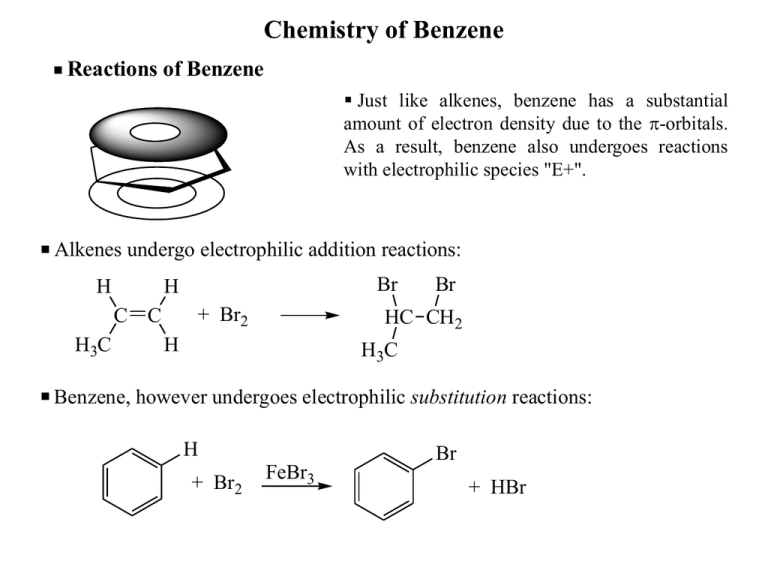
Chemistry of Benzene Reactions of Benzene Just like alkenes, benzene has a substantial amount of electron density due to the p-orbitals. As a result, benzene also undergoes reactions with electrophilic species "E+". Alkenes undergo electrophilic addition reactions: H + Br2 C C H3C Br H Br HC CH2 H H3C Benzene, however undergoes electrophilic substitution reactions: H + Br2 FeBr3 Br + HBr Electrophilic Aromatic Substitution (EAS) The general mechanism for all electrophilic aromatic substitution reactions is the following 2-step for process: The general mechanism ALL electrophilic aromatic substitution reactions is the following two step process: B H + H—B H E + H E E H -complex H The -complex is a very important intermediate. It is stabilized by delocalization of the positive charge. The -complex is a very important intermediate. It is stabilized by delocalization of the positive charge: H E H H E H H E H Electrophilic Aromatic Substitution Substitution vs. Addition: Substitution vs Addition Br H Br+ H H Br H -complex -complex Br H Br+ H H E H Br H Br -complex -complex E n er g y E ne rg y E HBr Reaction Progress Reaction Progress Substitution Addition H H Br Br Electrophilic Aromatic Substitution – Electrophiles E+ EAS reactions differ only in the identity of E+ and + how it is generated!! EAS reactions differ only in the identity of E and how it is generated. Nitration + HNO3 H2SO4 heat N O H + H2SO4 Reduction of nitroaromatics gives anilines. O O O NO2 H N O O In the case of nitration, E+ is NO2+. H O + HSO4 - N O + H2O Electrophilic Aromatic Substitution Sulfonation + SO3 SO3H O O S O O H2SO4 heat S OH + H2SO4 O + HSO4 - Electrophilic Aromatic Substitution Halogenation Br Fe or FeBr3 + Br2 + Cl2 Fe or FeCl3 Br Br + FeBr3 + HBr Cl + HCl + Br Br FeBr3 Electrophilic Aromatic Substitution H H H3PO4 H H O H3C C H H+ HO CH3 HO HO HO HO Bisphenol A HO OH Friedel-Crafts Alkylation Alkylation of Aromatic Compounds – The Friedel-Crafts Reaction: Alkylation of Aromatic Compounds - The Friedel-Crafts Reaction + RCl H3 C CH Cl R AlCl3 + AlCl3 H3C + HCl H3 C + CH Cl H3C AlCl3 H3 C H + AlCl4 C H3C When a relatively stable carbocation In some alkylations, this complex is possible, then stable it is likely the electrophile may In some alkylations this complex may When a relatively carbocation is serve as the alkylating electrophile. serve as the alkylating electrophile. possible, then it is likely the electrophile. Again, the mechanism for this reaction is no different than any other Electrophilic Aromatic Substitution. Cl H3 C H3C H C H CH3 CH CH3 The mechanism is the same as all other EAS reactions CH3 CH CH3 + HCl Friedel-Crafts Alkylation Carbocation rearrangements can occur during Friedel-Crafts Alkylations: Carbocation Rearrangement during Friedel-Crafts Alkylations + CH3 CH2CH2Cl CH2 CH2 CH3 AlCl3 CH3 CH 40% 35°C 5h CH3 60% PhH PhH H H3CH 2CH2 C Cl + AlCl3 + H3C C C H2 H Cl Rearrangements like this always a problem whe more stable carbocation result from a hydride or a shift. AlCl3 H3 C C H + AlCl4 H3C Such rearrangements always compete when a more stable carbocation can result from a hydride or alkyl shift. Friedel-Crafts Acylation Friedel-Crafts Acylation O H3 C C Cl O C AlCl3 O CH3 O H3 C C Cl + AlCl3 AlCl3 H3 C C+ Cl An acid chloride. Mechanism H3 C C O O O C H3C Cl H C O CH3 H3 C C ClO+ HCl + HCl H3 C C O The Acylium cation, a resonance stabilized carbocation, is the electrophile. CH 3 The Friedel-Crafts acylation can be used to circumvent the problems of carbocation rearrangement. O O H2NNH 2, KOH C AlCl3 CH2CH3 (Wolff-Kishner reduction) H 3CH2 C C Cl or Zn/Hg amalgam, HCl (Clemmenson reduction) CH2 CH2 CH3 The Friedel-Crafts acylation can be used to circumvent the problems of carbocation rearrangement. Reactions of Substituted Benzenes Electrophilic Aromatic Substitution of Substituted Molecules CH3 CH3 CH3 CH3 NO2 HNO HNO 3, 3 H 4 Acetic2SO Acid + Toluene 2-Nitrotoluene Statistical Statistical Actual Actual 40% 40% 60% 60% + NO2 NO 2 3-Nitrotoluene 4-Nitrotoluene 40% 40% 20% 20% 3% 3% 37% 37% This reaction is faster than the corresponding nitration of benzene. Why? And what is the reason for this particular product distribution? groups and several other This reaction is faster than the corresponding reactionAll of alkyl benzene. Why? substituents show this pattern of reactivity. NO2 HNO HNO 3 ,3 HH 2SO 44 2SO Nitrobenzene Statistical Actual NO2 NO2 NO2 NO2 + NO2 1,3-Dinitrobenzene 93%40% Yield 93% NO2 40% 20% Minor products trace trace This reaction is much slower than the nitration of benzene. Toluene gives mainly ortho and paraisproducts nitrobenzene gives the meta product almost exclusively. What This reaction slower while than the corresponding nitration of benzene. Why? is it about the substituent that directs the position of the incoming electrophile? Electrophilic Aromatic Substitution of Substituted Molecules Cl Cl NO2 HNO3, HNO 3 SO4 HH2SO 2 + + 4 30% o-Chloronitrobenzene Statistical Cl Cl 40% NO2 1% m-Chloronitrobenzene 40% NO2 69% 20% p-Chloronitrobenzene This reaction is slower than the nitration of 30% Actual 1% 69% benzene. All of the halogens follow this pattern of reactivity. What's going on? This reaction is slower than the corresponding nitration of benzene. Why? Why do toluene and chlorobenzene give predominantly ortho and para substitution, while nitrobenzene gives predominantly meta substitution? Electrophilic Aromatic Substitution - Activators Substituent Directing Effects -Complex Intermediates CH3 CH3 + NO2 H CH3 O N+ O + CH3 CH3 + NO2 H CH3 NO2 + CH3 CH3 NO2 H + NO2 H CH3 + NO2 H CH3 CH3 NO2 H CH3 NO2 CH3 + + + H NO2 H NO2 H NO2 NO2 Any substituent capable of stabilizing an adjacent positive charge (resonance or inductive) gives predominantly ortho and para substitution. Electrophilic Aromatic Substitution – Resonance Activators : : This includes ANY ATOM THAT CONTAINS A LONE PAIR and is directly attached to the ring. + OH : OH + H E Examples of this type of substituent: Br : : Cl : : : F : : : : : .. and thethe halides: …and halides: : OH NHR O O C R O N C R H : : : : : : OR NR2 : NH2 : Z= : Z : E I : H Resonance Activator Electrophilic Aromatic Substitution - Deactivators -Complex Intermediates O O- N+ + O NO2 O N+ O NO2 H ON+ NO2 H + O O- N+ O N+ ONO2 H O O+ N O O+ N + + O + O- N+ O- N+ NO2 NO2 + NO2 H O NO2 H NO2 NO2 H O O- N+ NO2 NO2 + + H NO2 + H NO2 H NO2 NO2 Any substituent which destabilizes an adjacent positive charge gives predominantly meta substitution. Electrophilic Aromatic Substitution - Deactivators ANY SUBSTITUENT WHICH DESTABILIZES AN ADJACENT POSITIVE CHARGE WILL BE A META-DIRECTOR. All of the following are meta-directors: O C O Esters OR C O O C C R Aldehydes Ketones Amides NR2 O C OH Carboxylic Acids C Nitriles O Cl Acid Halides S OH O Sulfonic acids NH3 N Electrophilic Aromatic Substitution of Substituted Molecules Directing Groups and Reaction Rate We have seen how substituents can be placed into two classes: those that are ortho/para-directors and those that are meta-directors. What about the effect of substituents on the rate of reaction? We made the following observations: 1.) Benzenes substituted with ortho/para-directors will react more quickly than benzene itself UNLESS THE SUBSTITUENT IS A HALIDE (F, Cl, Br, I). 2.) Benzenes substituted with meta-directors ALWAYS REACT MORE SLOWLY THAN BENZENE ITSELF. Why? Why? Recall that these substitutions are ELECTROPHILIC, i.e. the attacking species are attracted by the large amount of electron density circulating in the aromatic ring. + CH3 Alkyl groups are "electron releasing" or "electron donating". This is how they are able to stabilize adjacent positive charges in EAS reactions and in other carbocation species. (Recall that 3° carbocations are more stable than 2° carbocations because they bear more alkyl substituents.) Electrophilic Aromatic Substitution of Substituted Molecules Substituents with lone pairs on them can delocalize an electron pair into the aromatic ring thus dramatically increasing the electron density. This is reflected in the resonance structures for phenol. : + OH _ + OH : + OH : : + :OH _ _ In both these cases, electron density is donated by the substituent into the aromatic ring. Substituents that are capable of doing this are called ELECTRON DONATING GROUPS. By donating electron density into the aromatic ring they increase the charge density of the aromatic cloud making it more attractive to attacking electrophiles. These groups are therefore said to be ACTIVATING GROUPS as well as being ortho/para directors. Electrophilic Aromatic Substitution of Substituted Molecules _ O + N + : : Cl : O Meta-directors are electron withdrawing groups. They suck electron density away from the aromatic electron cloud making it less attractive to electrophiles. This slows the reaction between the electrophile and the aromatic ring as a result. Thus, meta-directors are said to be DEACTIVATING. What about the halides? Well, the halides strike a unique balance. They are ortho/para-directors because their lone pairs are capable of stabilizing an adjacent positive charge in a p-type of interaction. At the same time, the halogens are electronegative enough to diminish the electron density of the ring through the -bond.. + Electrophilic Aromatic Substitution of Substituted Molecules Directing influence o,p o,p o,p o,p m m Summary Substituent -NH2 , -NHR, -NR 2 (amino and aminoalkyl), -OH (hydroxy) Effect on Rate Very Strongly Activating -NHCOR (amide), -OR (ether), -OCOR (ester - O end) Strongly Activating R (alkyl), Aryl, Vinyl Activating X = F, Cl, Br, I (Halides) -CHO (aldehyde), -COR (ketone), -CO2H (carboxylic acid), -CO2R (ester), -COCl (acid halide), -CN (nitrile), -SO3H (sulfonic acid) -NO2 (nitro) Deactivating Strongly Deactivating Very Strongly Deactivating Functional Group Interconversions of Benzene substituents O C H2 C NO2 SO3H H2NNH2, KOH or Zn/Hg, HCl H2 C O KMnO4, or K2Cr2O7 C Pd/H2 or Sn/HCl aq. H2SO4 NH2 H OH Functional Group Interconversin of Benzene Substituents H The Sandmeyer Reaction I N Cl NH2 N NaNO2, HCl, < 5°C KX, Cu2X2 X = Cl, Br CuCN CN KI H3PO2 X BF4 H2O, HSO4, ² OH F