Experiment 8 Optical Isomers
advertisement

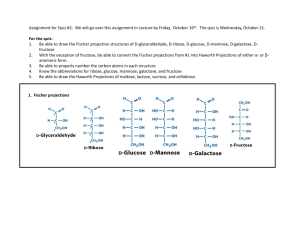
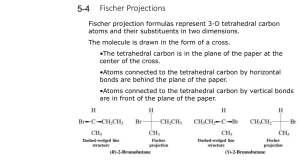
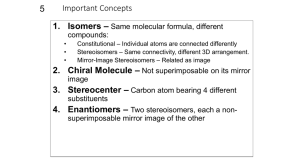
Experiment 8 Optical Isomers In this experiment you will be given the opportunity to see the 3-dimensional aspects of stereochemistry and optical isomers. Previously in class you were exposed to the concept of stereoisomers when you studied cis- and trans- geometric isomers for alkenes and di-substituted ring structures. Simply put, stereoisomers are isomers that have the same molecular and structural formulas but different spatial arrangements of their atoms. The following examples from that previous encounter are shown below: H3 C C C H CH3 H H C C H cis-2-butene Br H3 C CH3 trans-2-butene Br Br Br cis-1,3-dibromocyclopentane trans-1,3-dibromocyclopentane Chemists have developed methods to facilitate the visualization of 3-dimensional spatial arrangements of atoms or groups of atoms in a 2-dimensional environment, i.e., the plane of this paper. The most common method for depicting 3-dimensional structures in a 2-dimensional plane is the Fischer projection. Fischer projections are formed when the observer orientates a tetrahedral structure such that the atoms or group of atoms in the vertical plane are away from the observer (dashed wedges) while the atoms or group of atoms in the horizontal plane are towards the observer (solid wedges). The following diagram illustrates this concept: W C W X C Y X observer Y Z Z Therefore whenever a Fischer projection is seen it is meant to represent the following orientation of atoms or groups of atoms attached to the central tetrahedral carbon: W Z W X Y = Z C X Y Fischer Projection Note: only chiral carbons get the “cross” designation in a Fischer projection; all achiral carbons are shown as C. Utilizing this convention, you can now communicate 3-dimensional information of a molecule on a 2-dimensional surface. To further communicate information about stereoisomers several terms are used. Enantiomers are two non-superimposable mirror images and diastereomers are two non-superimposable, non-mirror images. Meso compounds are compounds that contain more than one chiral carbon but are optically incactive because of the symmetry of the molecule; the mirror images of these compounds are superimposable. Typically a meso compound can be identified by noting that it’s Fischer projection has a mirror plane, i.e., the top and bottom halves of the Fischer projection are mirror images of each other. Procedure: As before, use just the sticks to represent the bonds to hydrogens, the red balls for oxygen atoms, and green balls for nitrogen atoms. 1. Build the structures of D- and L- glyceraldehydes. Draw their Fischer projections such that that the aldehyde groups are at the top of your structures. Label the two isomers. What type of stereoisomers do these two structures represent? 2. Build the structures of the four stereoisomers for 2, 3, 4-trihydroxybutanal such that the aldehyde groups are at the top of your structure and the hydrogens and hydroxyl groups attached to chiral carbons point towards you. Draw their corresponding Fischer projections. Using your Fischer projections for D- and L-glyceraldehyde as a reference compound, label each of these Fischer projections using numbers, e.g., D1, L1, etc. What are the relationships between the various pairs of models? 3. Build the structures of all of the stereoisomers for asparagine such that the carboxylic acid group is at the top of your structure. Draw their corresponding Fischer projections. Label the isomers as D- or L-asparagine based on the D- or L-glyceraldehyde reference structures. In this case the carboxyl group and the a-amino group are analogous to the aldehyde and hydroxyl groups, respectively, of the reference compounds. O O HOC CH CH2 C NH2 NH2 asparagine 4. Build the structures of all of the stereoisomers for tartaric acid. How many unique structures did you build? Draw the corresponding Fischer projections. Indicate chiral centers with an asterisk *. Circle the structure that would not be optically active. O O HO C CH CH C OH OH OH tartaric acid 5. Build a model for 2, 3, 4, 5, 6-pentahydroxyhexanal such that the aldehyde group is at the top of the molecules and the hydroxyl group on the last chiral carbon (furthest from the aldehyde group) is pointing to the right, thus generating a D-structure for an aldohexose sugar. Also build your model such that the hydrogens and hydroxyl groups on each chiral carbon are orientated towards you. Draw the Fishcer projection corresponding to your model. Indicate chiral centers with an asterisk *. How many chiral carbons are there? Is the model you constructed straight or coiled? If coiled, is the hydroxyl group of the last chiral carbon nearby the aldehyde group? What type of compound would be formed if this hydroxyl group were allowed to react with the aldehyde group? Note- eight different stereoisomers with this D- designation can be made, one of which is the very important monosaccharide, D-glucose. Show your model and Fischer projection to your instructor. Compare your Fischer projection with the 8 possible D-hexose sugars and determine which sugar you have. Experiment 8: Optical Isomers Name ________________________ 1. Draw and label the Fischer projections for glyceraldehydes: 2. Draw and label the Fischer projections for 2,3,4-trihydroxybutanal: How many chiral centers are there? ________ How many total stereoismers are there? ________ How many pairs of enantiomers are there? ________ How many pairs of diastereomers are there? ________ 3. Draw and label the Fischer projections for asparagines: How many chiral centers are there? ________ How many total stereoisomers are there? ________ What would be a general formula for determining the maximum number of stereoisomers when n = number of chiral centers? 4. Draw and label the Fischer projections for tartaric acid, label all chiral centers with an *, circle the Fischer projection and draw its mirror plane for the meso compound. How many chiral centers are there? ________ How many total stereoisomers are there? ________ How many pairs of enantiomers are there? ________ How many pairs of diastereomers are there? ________ 5. Draw the Fischer projection for your 2, 3, 4, 5, 6-pentahydroxyhexanal and label all chiral centers with an *: How many chiral centers are there? _______ Based on your general formula, how many total stereoisomers are possible? _______ Show your Fischer projection to your instructor, which D-aldohexose did you construct? ____________________ Is your structure straight or coiled? ________ What class of compounds would be made if the hydroxyl group on carbon 5 reacts with the aldehyde group? _________ For the resulting cyclic structure how many atoms are in the ring? ________