403 The rhodamine derivatives tetramethyl-rhodamine-5/6- maleimide (TROMI) and tetramethyl-rhodamine-6-iso-
advertisement

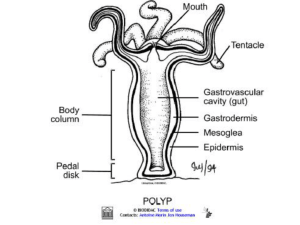
403 Journal of Cell Science 108, 403-412 (1995) Printed in Great Britain © The Company of Biologists Limited 1995 Novel tools for the study of development, migration and turnover of nematocytes (cnidarian stinging cells) Jakob Weber Zoological Institute, University of Zurich-Irchel, Winterthurerstr. 190, CH-8057 Zurich, Switzerland SUMMARY The rhodamine derivatives tetramethyl-rhodamine-5/6maleimide (TROMI) and tetramethyl-rhodamine-6-isothiocyanate (TRITC) were allowed to react with living Hydra vulgaris. The two fluorescent dyes stain the polyps to different degrees, apparently without impairing their viability and behaviour. Concerning nematocytes, TROMI preferentially couples to cytoskeletal elements only of mounted nematocytes whereas TRITC selectively reacts with structural components of cysts of late nematoblasts, which thereafter develop apparently normally into mature nematocytes. Hence TROMI-labelling indicates that nematocytes are mounted and ready for discharge; TRITC- labelling can be used as a tool to investigate the final maturation, migration and installation of nematocytes in Hydra. Together with a new non-fixative method to dissociate Hydra polyps into single, identifiable cells, the two labelling methods allow direct quantitative dynamic studies of nematocyte turnover and open new possibilities of investigating the regulation and the mechanisms of nematocyte supply and migration. INTRODUCTION scattered along the whole animal and are most abundant in the tentacles. The formation of nematocytes begins with the proliferation of committed stem cells (interstitial cells) located in the ectoderm of the body column to nematoblasts; it continues with the genesis and assembly of the complex intracellular cyst, the migration to and installation at their ultimate destination and, finally, ends with the discharge of the cysts into prey or aggressors, or displacement by new cells (for reviews see Campbell, 1988a,b; Bode, 1988). Studies on the overall temporal succession of the events in the nematocytes’ life (David and Gierer, 1974), morphological studies on the early development of nematoblasts (Slautterback and Fawcett, 1959; Holstein, 1981) and descriptive reports on nematocyte migration (Günzl, 1971; Campbell and Marcum, 1980), and distribution and turnover (Zumstein, 1973; Herlands and Bode, 1974; Bode and Flick, 1976; Weber et al., 1978), have been published. However, the methods used in these studies, namely, the direct observation of nematocysts in intact tissue, the radiolabelling of nematocytes and the maceration of tissue, which abolishes subcellular fine structures, limit the extent of such investigations considerably. In addition, some studies took advantage of grafting with epithelial (i.e. interstitial cell-free) Hydra, which, though elegant and illustrative, does not reflect the real situation. Therefore detailed insights into developmental or regulatory aspects (see Bosch and David, 1991), and mechanisms involved in migration (see González Agosti and Stidwill, 1991, 1992), as well as into the underlying principles of the regulation of genesis and life of all four types of nematocytes occurring in Hydra, are mostly Nematocytes, the stinging cells of Cnidaria (polyps, jellyfish, corals), are often characterised as the most elaborate and complex cells found in multicellular organisms. They occur in large numbers and many variants in all species of the Phylum Cnidaria and serve mainly for food capture and defence against aggressors (for reviews see Blanquet, 1977; Mariscal, 1974, 1984; Burnett and Calton, 1977, 1987; Tardent et al., 1985; Hessinger and Lenhoff, 1988; Williams et al., 1991). For several reasons nematocytes and their progenitors, the nematoblasts, are excellent objects, not only for studying toxinological, mechanistic and signal transductional aspects of the exocytotic discharge of nematocysts but also for investigating more general cell biological phenomena of multicellular organisms, particularly of the control and the mechanisms of cell differentiation, of cell migration or the regulation of cell numbers at the level of the live and intact organism: the differentiation pathway of nematocytes is well defined and not very long, nematocytes are relatively easy to observe and manipulate in vivo and can, due to their prominent intracellular capsule (the nematocyst), be identified rather easily amongst other cells. The organisms in which nematocytes occur are relatively primitive and therefore in some respects easier to study than, e.g. the more highly evolved insects or vertebrates. In addition, some freshwater and marine cnidarians can easily be kept in large numbers under laboratory conditions. The freshwater polyp Hydra vulgaris (Campbell, 1989) consists of a closed diploblastic body column with a gastric cavity and a crown of tentacles. Nematocytes are found Key words: Hydra, nematocyte, nematocyst, development, migration, covalent labelling, rhodamine 404 J. Weber lacking. Even less is known about nematocytes from other representatives of the Cnidaria. In this paper methods for the selective in vivo fluorescentlabelling of developing and of mounted nematocytes are presented. Furthermore, a new and convenient method to dissociate Hydra polyps under non-fixative conditions into single cells is introduced. These methods allow the direct study of the development of later stages of nematoblasts and the turnover of nematocytes. MATERIALS AND METHODS Materials Cultures of Hydra vulgaris (Hydrozoa; Cnidaria) (Campbell, 1989) were grown in culture solution as described (Loomis and Lenhoff, 1956; Weber et al., 1987) and fed four times per week with Artemia larvae. For all experiments and analyses randomly chosen specimens from these mass cultures were used. All reagents used were of analytical grade. Dissociation of Hydra polyps into single cells A small volume of culture solution containing 3 to 10 Hydra polyps at 14°C was pipetted into 1 ml of dissociation medium (see below), preheated to 40°C. After reducing the total volume to approx. 60 to 200 µl, the polyps were incubated for 10 minutes at 40°C and subsequently dissociated mechanically to form a suspension of single cells (or cell clusters) by vigorously shaking the tubes. The degree of dissociation was controlled visually; too-intense shaking resulted in an increase in cell fragments, too-gentle shaking yielded larger tissue pieces. The dissociated cells were held on ice and examined under a Reichert-Jung Polyvar microscope equipped with Nomarski differential interference (DIC) optics. Pictures of individual cells as presented in Fig. 1 were recorded with a video camera mounted on a Polyvar microscope and transferred on-line to a Macintosh computer. The pictures were processed and arranged with the software program Adobe Photoshop 2.5.1. The composition of the dissociation buffer, which was routinely used for identification and/or counting of different stages of nematoblasts and nematocytes, was 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.2 (PBS). In some experiments a dissociation medium, made according to Gierer et al. (1972), and supplemented with 54 mM KCl, was used to improve the (osmotic) stability of the epithelial cells. If large polyps have to be dissociated it is sometimes advisable to cut the animals into smaller pieces prior to the 40°C incubation, since the endodermal cells are held tightly together by the massive acellular mesoglea. In vivo labelling of Hydra with TRITC or TROMI Hydra polyps were incubated in Hydra culture solution including 1 µM tetramethyl-rhodamine-6-isothiocyanate (TRITC; Molecular Probes) or 25 µM tetramethyl-rhodamine-5/6-maleimide (TROMI; Molecular Probes) for 30 minutes at room temperature. These concentrations proved to be optimal for our purposes and apparently did not affect the polyps’ health (see Results). After washing the polyps by repeatedly transferring them into new, dye-free culture solution, they were kept and fed as described for untreated animals. Stock solutions (1 mM) of TRITC and TROMI in water were stored frozen at −20°C up to several weeks without an observable reduction in the chemical reactivity. They were diluted with Hydra culture solution immediately before use. Analysis of nematoblasts and nematocytes in labelled Hydra Isolated nematocysts After different periods of time after TRITC-labelling about 20 intact Hydra (or polyps separated into tentacles including hypostome and body column excluding buds) were frozen at −20°C in a small volume of water. Cell-free nematocysts were isolated from these frozen probes as previously described (Weber et al., 1987; Weber, 1990) using 70% Percoll (Pharmacia). The purified nematocysts were pipetted onto glass slides, dehydrated in a vacuum desiccator and stored in a dry state. The amounts of labelled and unlabelled nematocysts of the different types were determined after mounting the isolated cysts in 40% poly(ethylene glycol) 1550 (PEG; Serva), 5% propyl gallate (Fluka; Giloh and Sedat, 1982), 2 mM Tris-HCl, pH 9, under a Reichert-Jung Polyvar microscope equipped with Nomarski-DIC and epifluorescence illumination (a filter set designed for rhodamine fluorescence was used). Addition of PEG to the mounting medium permits discrimination between fully mature and immature nematocysts, since cysts that are not yet under the full internal osmotic pressure shrink anisotropically and display irregularities in their shape (see Weber, 1989). Totals of 300 to 500 undischarged and mature nematocysts of each type (stenoteles, desmonemes, isorhizas) were counted and classified as labelled or not. The criterion for a cyst as being labelled was in all three cases a clearly identifiable fluorescent internal tubule (see Results). Single nematocytes TRITC- or TROMI-labelled Hydra were dissociated into single cells as described above. After removing most of the epithelial cells by a 2 minute centrifugation at 2,000 g in 50% Percoll in dissociation buffer (0°C), the remaining, sedimented cells (mostly interstitial cells, nematoblasts, nematocytes, some gland cells) were immediately analysed as a cell suspension in dissociation buffer and classified as outlined above for isolated nematocysts (100 to 200 stenoteles and isorhizas were routinely counted). Since some late-stage immature nematocytes are difficult to distinguish optically from mature nematocytes, the counts may include some immature nematocytes (see Results). TROMI-labelled cells were identified by their highly fluorescent bundles of rods (see Results). In some experiments tentacles and body columns were analysed separately. Intact tissue Qualitative studies with TRITC- or TROMI-labelled Hydra were performed after fixation for 10 minutes in absolute ethanol. The fixed polyps were gradually rehydrated, their pedal disks and tentacle rings were dissected, the body column was opened with fine tungsten needles and the endoderm stripped off the body column and discarded. Tentacles and body column ectoderm were mounted in PEG-propyl gallate-Tris (see a above) and examined in the fluorescence microscope. The fixed animals were studied within 2 hours following fixation, since the distribution of the fluorescence changes slightly within hours after fixation. Ethanol fixation proved to be superior to other fixation methods, since the discharge of nematocysts is minimal. Transplantation of Hydra polyps At 0, 24 or 48 hours after TRITC labelling, the distal (upper) halves of unlabelled polyps were grafted onto the proximal (lower) halves of labelled polyps (Tardent, 1966). Only bud-less polyps were used. After 24 or 48 hours of parabiosis, the polyps were fixed for 10 minutes in absolute ethanol, gradually rehydrated and then mounted in PEG-propyl gallate-Tris and examined microscopically for fluorescent nematocytes that had migrated into the unlabelled tissue. RESULTS Dissociation of Hydra into single cells Hydra polyps can be macerated (David, 1973) and the various types of cells can be easily identified, classified, counted and Novel tools for studying nematocytes autoradiographed. However, intracellular organelles and fine structures are disrupted; furthermore, different stages of nematocyte development are difficult to identify and mature nematocysts may lose some of their content (Weber, 1994). On the other hand, if polyps are dissociated (Gierer et al., 1972), the viable single cells obtained are very fragile and not suited for quantitative studies or fractionation (see Greber et al., 1992). Therefore, the development of an alternative, gentle method for dissociating polyps rapidly and quantitatively into single cells was desirable. Incubation of Hydra in salt solutions of moderate ionic strengths (see Materials and Methods) at elevated temperatures (routinely 40°C) for a few minutes permits the dissociation of polyps (including their tentacles) into single cells and/or, depending on the intensity of the mechanical disruption, into groups of tightly associated cells such as battery cells or clusters of nematoblasts (Fig. 1). The different cell types of Hydra (Bode et al., 1973; David, 1973) are easily identifiable and observed at amounts equivalent to those obtained by maceration; only nerve and sensory cells were more difficult to identify, since after rapid (partial) retraction of their processes they become, in many cases, indistinguishable from interstitial stem cells. Fig. 1 shows a collection of abundant cell types after the dissociation of whole polyps; nematocytes and different developmental stages of nematoblasts can readily be identified and quantified. Under the conditions used, the dissociated cells are subvital; 405 i.e. despite frequently observed vigorous active shape changes, they cannot reaggregate and regenerate new polyps (Gierer et al., 1972; Flick and Bode, 1983). Within the first hours after dissociation, however, their intracellular topological organisation and physiology seem not to be grossly affected. By raising the temperature during dissociation to 50°C, cells are obtained in more ‘fixed’ state, and by using salt solutions of different compositions, the (osmotic) stability of particular cell types can be optimised. Cells are kept for at least 2 hours in a good state and were, for example, fractionated within this period by density gradient centrifugation in Percoll-containing dissociation buffer. By this procedure tentacles are dissociated into single endodermal epithelial, ectodermal battery (ba in Fig. 1) and nerve/sensory/interstitial cells. After more vigorous dissociation, nematocytes (is, st and de in Fig. 1) are gradually lost from the battery cells and under the conditions used here, most of the stenoteles (approx. >80 to 90%) are no longer found within the battery cells. Labelling of living Hydra by rhodamine B derivatives The two rhodamine derivatives tetramethyl-rhodamine-isothiocyanate (TRITC) and tetramethyl-rhodamine-maleimide (TROMI) were chosen for covalent labelling of Hydra (see also Klimek, 1979). TRITC (1 µM) added to Hydra culture solution, diffuses Fig. 1. Collage of dissociated cells of whole Hydra. Polyps were dissociated and the single cells were recorded as described in Materials and Methods. The selected cells shown are identified as ectodermal (ec) and endodermal (en) epithelial cells, gland cell (gl), nerve cell (ne), tentacular battery cell (ba), pair of i-cells (pi; presumably stem cells), and different stages of nematoblasts (nb1: 16- or 32-cell cluster of very early nematoblasts; nb2: part of a more developed cluster, presumably of desmonemes; nb3 and nb4: parts of clusters of later stages of stenotele nematoblasts) and the nematocytes, stenotele (st), desmoneme (de), holotrichous isorhiza (is). 406 J. Weber readily across cell membranes and couples at varying degrees to most of the tissue components of both the ecto- and the endoderm of polyps. There is no evidence that the animals are affected negatively in their behaviour or morphology by the procedure; even several months after labelling the polyps still fluoresce to a considerable degree. In certain populations of nematoblasts the growing cysts are strongly and selectively labelled by TRITC: late-stage nematoblasts (see below) display highly fluorescent walls and shafts, tubules and adhering structures, whereas in earlier nematoblasts as well as in mature nematocytes only a negligible amount of the reactive dye couples to the cyst’s wall. TRITC-labelled nematoblasts develop and mature normally and apparently without delay into fully functional nematocytes. TROMI (25 µM) in contrast to 1 µM TRITC, labels living Hydra only weakly, and most of the incorporated fluorescence Fig. 2. Labelling of Hydra with TROMI. Hydra were labelled with TROMI and subsequently fixed and mounted as described in Materials and Methods. The plane of focus for the fluorescence pictures (upper row) lies close to the apical part of the cell surface of the nematocytes, and the focus of the corresponding Nomarski-DIC pictures (lower row) was adjusted to deeper within the tissue in order to be able to identify the types of nematocysts. (A) Four battery cells in a tentacle (with numerous desmonemes, some isorhizas and 3 of them with a prominent stenotele as indicated by the arrowheads); and (B) parts of the body surface (with some holotrichous isorhizas that are mounted perpendicular to the plane of focus and 2 stenoteles, which are marked by arrowheads). Bar, 20 µm. is detected associated with presumably endosomal compartments of ectodermal epithelial cells. As with TRITC, the animals survive without behavioural or morphological anomalies. The most striking feature with TROMI labelling is its selective reaction with cytoskeletal elements of mature nematocytes that are in direct contact with the outer milieu, i.e. with those nematocytes that are mounted. If the labelling concentration of TRITC is raised to 5 µM and higher, the staining becomes more intense than with 1 µM dye, but the polyps show temporary degenerative effects; TROMI seems to affect the polyp’s health only at concentrations exceeding 100 µM. If ethanol-fixed and thus permeabilised Hydra are reacted with TROMI, a pronounced and general increase in the fluorescence of the tissue, as compared to the in vivo labelling, is observed. In contrast, TRITC labelling in living and fixed Novel tools for studying nematocytes polyps is quantitatively and qualitatively similar. The different staining properties of TRITC and TROMI, therefore, seem to be based on the different potencies of these reagents to cross cellular membranes and not on their different intrinsic chemical reactivities (TRITC preferentially reacts with amines, TROMI with sulfhydryls). The pronounced selectivity of TRITC for later nematoblast stages, in contrast, obviously reflects a higher chemical reactivity of the cysts of these cells as compared to the cysts of earlier nematoblasts or mature nematocytes. Labelling with TROMI Fig. 2 shows parts of a tentacle (A) and of a body column (B) of TROMI-labelled Hydra polyps under fluorescence and Nomarski-DIC illumination. In the tentacle as well as in the body column, those nematocytes that are on the surface of the tissue (and are thus presumably mounted) show highly fluorescent cnidocils (sensory cilia) and intracellular basket-like structures. The fluorescence labelling pattern is retained after dissociation of the polyp into single cells (not shown) and is stable for at least seven days. The labelled intracellular structures correspond to the so called rods, uncharacterised integral components of the tubulin and actin polymer-containing complex basket that envelopes the nematocysts (Stidwill and Honegger, 1989). The functionality of the nematocytes seems not to be affected by the labelling. During the course of this work, immediately after TROMI labelling, neither fluorescent nematocytes apart from the surface of the polyp nor non-fluorescent nematocytes that were obviously mounted were found. The relative amount of labelled nematocytes in the body columns of Hydra was determined by dissociating polyps immediately after the TROMI incubation and counting the amount of fluorescent cells (Table 1). Labelled and thus mounted desmonemes or atrichous isorhizas were observed rarely, if at all, in agreement with published work (Bode and Flick, 1976). The amounts of labelled stenoteles and holotrichous isorhizas were determined as 26±6% and 85±6%, respectively, in the body columns (Table 1). Both values fluctuate considerably even within the same clone of Hydra (see inset of Fig. 3, and the broad ranges in Table 1), which indicates that the number of mounted nematocytes can vary extensively (see Zumstein, 1973; Bode and Flick, 1976). The amount of labelled nematocytes in the body column at various times after a single incubation with TROMI is shown in Fig. 3. For stenoteles and holotrichous isorhizas the labelling frequency initially decreases by about 20 to 30% per day and later about 10% per day compared with freshly labelled controls. These results are consistent with the assumption that TROMI labels only mounted nematocytes, which are then gradually lost within one to two weeks (Bode and Flick, 1976). Labelling with TRITC Fig. 4A to C shows different parts of the body column ectoderm of freshly TRITC-labelled Hydra by fluorescence and Nomarski-DIC illumination. Although most tissue components are fluorescent, nematoblasts in which the growing capsule itself is heavily labelled are clearly observable. Fig. 4A shows three fluorescent stenotele nematoblasts (triangles) out of a larger cluster, Fig. 4B various clusters of fluorescent desmoneme nematoblasts, and Fig. 4C two single, nearly mature, fluorescent stenotele nematoblasts (triangles). Mature nematocytes (see the single stenotele marked with an asterisk in 4A) and earlier stages of nematoblasts (see the two stenotele nematoblasts with asterisks in 4C), however, are strikingly non-fluorescent. A careful inspection of body columns and of dissociated single cells of labelled polyps confirms that neither very early stages of nematoblast nests nor fully mature nematocytes of any type display the typical brightly fluorescent pattern of later nematoblasts shown in Fig. 4A-C. In particular, no TROMI-positive and/or no tentacular nematocytes were found to be fluorescent immediately after the TRITC treatment. The ability to be labelled by TRITC seems to be restricted to clusters of nematoblasts of which the future capsule type is clearly identifiable and whose capsular wall has lost its pronounced fragility. This labelling ability is lost shortly after the disintegration of the nematoblast nests. Fig. 4D shows cell-free nematocysts isolated five days after labelling of living polyps with TRITC. Two stenoteles, two Nematocytes Stenoteles Desmonemes Holotrichous isorhizas Atrichous isorhizas Mean (%) s.d. (%) Range (%) Counts 26 <5* 85 <5* 6 16-38 23 6 75-94 16 Hydra were reacted with TROMI and the body columns of the polyps were dissociated as outlined in Materials and Methods, and examined with a fluorescence microscope. For each count 100 to 200 nematocytes of the indicated type were classified as labelled or not labelled. The data were collected over the range of two months. *Found only rarely and erratically; possibly cross-contaminations from tentacular tissue. AAAAAAAAAA AAAAAAAAAAAAAAAAAA AAAAAAAAAA AAAAAAAAAAAAAAAAAA AAAAAAAAAA AAAAAAAAAAAAAAAAAA AAAAAAAAAA AAAAAAAAAAAAAAAAAA AAAAAAAAAA AAAAAAAAAAAAAAAAAA AAAAAAAAAAAAAAAAAA AAAAAAAAAAAAAAAAAA AAA AAAAAAAAAAAAAAAAAA AAA AAAAAAAAAAAAAAAAAA AAA Relative amount of labelled 100 nematocytes in the body column (%) 80 100 60 40 80 60 40 20 0 0 2 4 6 Stenoteles holotr. Isorhizas 20 Table 1. Relative amount of TROMI-labelled nematocytes in the body column of Hydra 407 F F F F 0 0 1 2 3 4 5 Time after TROMI-labelling (days) 6 7 Fig. 3. Decrease in fluorescent nematocytes within several days after TROMI labelling. At 0, 1, 2, 5 and 7 days following incubation of Hydra with TROMI (see Materials and Methods), the body columns of 20 polyps were separated. Ten of them were used as controls and were additionally labelled with TROMI (filled symbols in the inset), the others were used directly (open symbols in the inset). After dissociation into single cells, the fractions of labelled stenoteles (squares) and holotrichous isorhizas (circles) were determined and plotted in the inset. The main graph was obtained by referring the obtained values to the respective controls, which were set to 100%. Hydra were fed where indicated (F). 408 J. Weber desmonemes and one isorhiza in this figure display highly fluorescent intracapsular structural components whereas in one stenotele only the surrounding wall is slightly fluorescent. The former have, without losing their fluorescence, obviously developed from labelled nematoblasts, whereas the latter was already mature at the time of the TRITC labelling (see also Fig. 5). In isolated mature nematocysts (Fig. 4D) the TRITC fluorescence is not abolished after incubation of the cysts in 1 M sodium hydroxylamine-hydrochloride, pH 8, or 0.5 M sodium glycinemethylester-hydrochloride, pH 10, indicating that the dye is not coupled via sulphydryl groups (as could be assumed from their high content in walls and tubules (Blanquet and Lenhoff, 1966; Kurz et al., 1991) but rather via amino groups (Drobnica et al., 1977)). The TRITC reaction is not a strict pulse-labelling, since hours to days after washing out the dye nematoblasts entering the critical, TRITC-susceptible stages become labelled to some degree, presumably by unreacted dye stored within the Hydra tissue. Furthermore, the fluorescence intensities of all labelled cysts become somewhat reduced during the final maturation phase by either loss or chemical derivatisation of the dye or by quenching (compare the fluorescence intensities of the two nearly mature stenoteles in Fig. 4C with the three earlier stages in Fig. 4A, which are all marked with triangles). Fig. 5 shows the time course of the appearance of mature fluorescent nematocysts at various periods of time after TRITC labelling of living Hydra. The amount of cysts that are fluorescent initially lies between 0 and 10% for each of the different types of cysts and increases gradually within the next days. An additional inspection within 1 hour after TRITC treatment indicates that in the case of the stenoteles and holotrichous isorhizas all fluorescent cysts display irregularities in their shapes if they are mounted in the high-osmotic PEG solution (see Materials and Methods). Obviously, their full intracapsular osmotic pressure is not yet established (Weber, 1989), and they must be considered as immature. This is also true for a small fraction of the cysts at later times. For the smaller atrichous isorhizas and desmonemes it is not clear whether the few fluorescent cysts found immediately after labelling (Fig. 5) are immature, but it is assumed that the situation is similar to that in the other two cyst types. Therefore, the ability of cysts to be labelled by TRITC is lost at exactly the moment when the full internal osmotic pressure is attained, i.e. the point at which the cysts become operationally mature and have acquired their high buoyant densities (Weber et al., 1987, 1988). The gradual increase in the relative amounts of fluorescent nematocysts within a few days, as illustrated in Fig. 5, supports the assumption that following TRITC treatment all of the newly maturing nemato- Fig. 4. Labelling of Hydra with TRITC. TRITC-labelled Hydra were fixed and mounted as outlined in Materials and Methods. The fluorescence pictures shown in the upper row correspond to the respective Nomarski-DIC pictures of the lower row. (A) to (C) Selected parts of the body column. The triangles indicate fluorescent late stenotele nematoblasts whereas asterisks mark a mature stenotele (A) and nonfluorescent stenotele nematoblasts (C) (see text). (D) Cell-free nematocysts (s, stenotele; i, holotrichous isorhiza; d, desmonemes) isolated from whole Hydra 5 days after TRITC-labelling. Bars, 20 µm (same magnification in (A) to C)). Novel tools for studying nematocytes AAAAAAAAAAAAAAAAA AAAAAAAAAAAAAAAAA AAAAAAAAAAAAAAAAA AAAAAAAAAAAAAAAAA AAAAAAAAAAAAAAAAA AAAAAAAAAAAAAAAAA AAA AAAAAAAAAAAAAAAAA AAA 50 Amount of labelled cysts in the whole polyp (%) 40 30 20 Stenoteles Desmonemes Isorhizas 10 0 0 20 40 60 Time after TRITC-labelling (hours) 80 100 80 60 40 20 cytes are fluorescent. As in the case of TROMI labelling, the rates of increase of TRITC-labelled nematocytes with time can vary considerably amongst different Hydra clones and even within the same clones (see also below and Fig. 6). The fate of TRITC-labelled nematoblasts Dynamic aspects of the nematocyte turnover were studied by analysing the body column and tentacles for the temporal appearance of fluorescent cysts after labelling Hydra with TRITC. Fig. 6 shows the time course of maturation of new (fluorescent) nematocytes and the displacement of old (non-fluorescent) ones in the body column (A) and the tentacles (B) of the polyps. Since only mature cysts (plus a small fraction of immature ones; see above) survive the preparation procedure before counting, the figure confirms that the ability of stenoteles and isorhizas to become labelled is lost when the full intracapsular osmotic pressure is completely established. Since the number of desmonemes in the body column is relatively low and the fraction of presumably immature but morphologically similar cells is rather high, the graph for the desmonemes is shown artificially shifted to too-high absolute values. As deduced from Fig. 6A new desmonemes replace old, unlabelled ones in the body column with a t50% of about 0.5 day. The latter are assumed to migrate into the tentacles (see above, and Bode and Flick, 1976). A t50% of about 1.0 day is determined for the about 70 to 80% of stenoteles that are not mounted in the body column (Table 1). The replacement velocity of these latter ones, which are mounted and ready for discharge, is much longer (t50% approx. 3 to 4 days; see Fig. 3). For the isorhizas the replacement rates cannot be estimated precisely, but the steep initial increase indicates that the replacement rates for the approx. 15% unmounted holotrichous isorhizas (Table 1) and the relatively low amount of atrichous isorhizas are probably similar to those of desmonemes. Fig. 6B illustrates the situation in the tentacles of the same polyps: labelled desmonemes and isorhizas migrate after maturation, apparently without any delay into tentacles, and their number increases there at a nearly constant rate of approx. 15% per day within the first 4 days. Stenoteles, in contrast, appear only after a lag phase of approx. 1.0 to 1.5 days after matura- A 0 Stenoteles Desmonemes Isorhizas B Amount of labelled cysts in the tentacles (%) 100 80 60 Fig. 5. Increase in labelled mature nematocysts after incubation of Hydra with TRITC. After the indicated periods of time following TRITC labelling, 20 whole polyps were frozen and used for the isolation of their nematocysts. For desmonemes (triangles), stenoteles (squares) and isorhizas (circles) the relative amount of fluorescent cysts was determined by counting. Hydra were not fed during this experiment. For details see Materials and Methods. AAAAAAAAAA AAAAAAAAAAAA AAAAAAAAAA AAAAAAAAAAAA AAAAAAAAAAA AAAAAAAAAA AAAAAAAAAAAA AAAAAAAAAAA AAAAAAAAAA AAAAAAAAAAA AAA AAAAAAAAAAA AAA AAAAAAAAAAA AAA AAAAAAAAAAA AAAAAAAAA AAAAAAAAAA AAAAAAAAA AAAAAAAAAA AAAAAAAAA AAAAAAAAAA AAAAAAAAA AAAAAAAAAA AAAAAAAAAA AAAAAAAAAA Amount of labelled cysts in the body column (%) 100 40 20 0 409 F F F 0 1 2 4 3 5 Time after TRITC-labelling (days) F 6 7 Fig. 6. Dynamic aspects of nematocyte maturation. To determine the increase in newly maturated nematocytes, nematocysts were isolated separately from body columns and from tentacles of Hydra 0 to 7 days after TRITC labelling and counted as described in the legend to Fig. 5. Hydra were fed where indicated (F). tion in tentacles. Thereafter their amount increases significantly faster than the amount of the other types of cysts. The data of Fig. 6 agree with assumptions of Bode and Flick (1976) that within about 9 days all the nematocytes of a tentacle become replaced by new ones, due to loss of cells at the distal end of the tentacles and proximal insertion of new battery cells; the even more rapid increase in stenoteles suggests that this type of cyst is used and therefore replaced due to intense food capture at much higher rates than the desmonemes and isorhizas. In further experiments, both, the fundamental time course of the graphs and the corresponding displacement rates, as well as the typical lag phase for the appearance of stenoteles within tentacles, were confirmed, although the absolute values may differ to some degree. The quantitative data of Fig. 6 could be sustained qualitatively by microscopic observation of nematocytes that migrated through the body column of polyps into the tentacles. For this purpose, distal parts of untreated polyps were grafted onto proximal halves of Hydra 0, 1 and 2 days after incubation in TRITC. Within the first day after labelling, high numbers of desmonemes and isorhizas but essentially no stenoteles had migrated to the unlabelled, distal part of the body column or into tentacles. Atrichous isorhizas were the first nematocytes to reach distal parts of the tentacles, followed by desmonemes and holotrichous isorhizas. After two days, in addition, numerous stenoteles were recorded in the upper body column and within tentacles (up to more than 10 new stenoteles per tentacle). This was observed both in transplants that had been grafted immediately after labelling (2 days of parabiosis) and in those grafted only one day after labelling (1 day of parabiosis). The pronounced increase in stenoteles migrating into tentacles between day 2 and day 3, as demonstrated in Fig. 6, could also be observed independently of the time of parabio- 410 J. Weber sis in the same type of experiment (1 or 2 days of parabiosis). These observations suggest that the delay of about one day for stenoteles appearing in tentacles (Fig. 6B) is based on the (apparent) lag-phase of stenotele development before starting to migrate and not on a relatively slow average migration velocity of these cells through distal (upper) parts of the body column. Like other types of nematocytes, stenoteles, after this lag phase, are able to migrate from lower parts of the body column up to the tips of tentacles within less than 0.5 day. In addition, it was found that the majority of desmonemes and isorhizas are mounted most proximally in the new, still empty battery cells of the tentacles as reported (Bode and Flick, 1976), whereas stenoteles are found more scattered along the whole length of the tentacles. This confirms the assumption (see above) that stenoteles discharge more frequently (and have to be replaced by new ones more often) than other cysts. DISCUSSION It has been shown previously that multipotent stem cells of the body column of Hydra that have been committed to nematocytes undergo three to five cycles of synchronous cell divisions and develop into mature nematocytes within about 5 to 7 (for desmonemes and isorhizas) or 7 to 8 (for stenoteles) days (David and Gierer, 1974). During this differentiation period the nematocysts grow within a modified Golgi vesicle via a highly sophisticated cascade of assembly processes to their final size and complex structure (Slautterback and Fawcett, 1959; Holstein, 1981). The mature nematocytes migrate within the ectodermal tissue to their final destination, the outer surface of the body column or, more frequently, the ectoderm of the tentacles. There the nematocytes are mounted and become susceptible to external stimuli that trigger their discharge (Watson and Hessinger, 1989; Golz and Thurm, 1991). Although the migration of nematocytes has been studied directly by microscopic observation in vivo (Günzl, 1971; Campbell and Marcum, 1980) or in vitro (González Agosti and Stidwill, 1991, 1992) as well as statistically (mainly with radiolabelled nematocytes) (Tardent and Morgenthaler, 1966; Zumstein, 1973; David and Gierer, 1974; Bode and Flick, 1976; Herlands and Bode, 1974; Weber et al., 1978), many aspects of the nematocyte movements remain unclear, and the overall knowledge of the processes of nematoblast development, not only in Hydra, is only marginal (see Campbell, 1988b; Kurz et al., 1991). The novel methods The new cell dissociation method presented here is based on a procedure for dissociating Hydra polyps into single cells, which are able to reconstitute a new polyp (Gierer et al., 1972; Flick and Bode, 1983), and on a similar protocol for dissociating marine cnidarians (Schmid et al., 1981). Compared to the Gierer method, the one presented in this article allows a more easy identification of the particular cell types, improved intrinsic stability of the dissociated cells and almost complete dissociation of body columns and tentacles without significant amounts of cellular debris. It enables us to use the method for quantitative work and to optimise the dissociation procedure (changes in the buffer composition, dissociation temperature) for particular purposes. Compared to David’s (1973) maceration procedure, which has succesfully been used mainly for quantitative analyses of whole Hydra or tissue fractions including autoradiography, the present dissociation method possesses the advantage of being non-destructive; it still permits the identification and analysis of subcellular structures. In addition to the results presented in this article, dissociated cells have succesfully been applied to flow cytometric cell sorting (Maurer, 1988), various autoradiographic studies, analyses of endocytotic events and the evaluation of selective fluorescent staining of subcellular compartments (all unpublished). There is good evidence that TROMI selectively labels those nematocytes of living Hydra that are mounted and thus ready to be triggered for discharge. This is demonstrated by microscopic inspection of labelled intact Hydra tissue and by quantitatively analysing the content and temporal decrease in fluorescent nematocytes from initially TROMI-labelled polyps. Whereas dye that is covalently coupled to cnidocils can in some cases be lost after regeneration of these fragile structures (Golz and Thurm, 1990), the characteristic labelling of the rods seems to persist for the rest of the nematocyte’s life span. The fluorescence of these uncharacterised structural components of the nematocyst’s enveloping cytoskeleton (Stidwill and Honegger, 1989) can thus be considered as an indicator that the particular nematocyte was mounted at the time of the TROMI application. It is not clear why TROMI, which generally seems to be unable to diffuse across cellular membranes of Hydra, reacts preferentially with the intracellular rods. Our observation might indicate that the (apical) cell membrane is in its physical properties somehow different compared to other cell membranes facing the outer milieu, but the preferences of the dye for the rods remain unexplained. However, the high and selective affinities of TROMI for these uncharacterized structural components of nematocytes could now be used as a tool to investigate their biochemical nature and physiological role. As shown by microscopical observations in Hydra tissue and by data obtained after isolation of intact nematocysts, TRITC couples covalently (in addition to many other Hydra tissue components) preferentially to cysts within nematoblasts that are just about to become mature. In contrast to the reaction with TROMI, here it is the cyst’s wall and the inverted tubule and its associated structures that couple to the dye, but it is again unclear what is the reason for this particular behaviour. Since a similar labelling pattern is observed in permeabilised, fixed Hydra, the labelling potency does not seem to be governed by membrane permeabilities. It may rather depend on temporal changes in the availability of reactive nucleophiles of nematocysts during the phase of their final maturation. In contrast to TROMI, TRITC is not a strict pulse-labelling agent but stains late-stage nematoblasts during at least six days after exposing the Hydra polyps to the dye, albeit at decreasing intensities. The staining properties of Hydra nematoblasts and nematocytes with TROMI and TRITC are not unique. It has been found (unpublished) that there is a similar staining behaviour with the pair of dyes N-(7-dimethylamino-4-methyl coumarinyl)maleimide (Yamamoto et al., 1977) and monobromobimane (Kosower et al., 1979) and it is to be expected that other dyes with similar properties can be found. Novel tools for studying nematocytes Turnover of nematocytes The suitability of the present novel methods for studies of maturation, migration and turnover of nematocytes is demonstrated by the results obtained, which extend established data (Zumstein, 1973; Bode and Flick, 1976). As outlined in Results the different types of nematocytes lose their ability to be stained by TRITC at the time when the final internal osmotic pressure of their cysts is attained. In the following, it will be considered as the time of becoming mature. Desmonemes are normally not mounted in the body column and, once mature immediately start to migrate to the tentacles. Their mean time of residence in the body column as mature cells is only approx. 0.5 day. Once in the tentacles the majority of them are mounted proximally in newly differentiated battery cells. Within about 4 days half of the tentacular desmoneme population is replaced by new ones. Atrichous isorhizas, which are rather difficult to observe because their number is low, seem to behave similarly to desmonemes. Holotrichous isorhizas are mounted mainly in the body column. They too start to migrate immediately after the final maturation. Preliminary observations indicate that these nematocytes are not mounted until 10 to 15 hours later. Holotrichous isorhizas that migrate into tentacles again seem to behave similarly to desmonemes. Stenoteles, in contrast to the other three types of nematocytes, appear only after a lag phase of 1 to 1.5 days after maturation in the tentacles. Their turnover rate there, however, is higher than either of the other types, presumably because they are consumed in higher numbers than the others. As revealed in preliminary observations, stenoteles are not mounted before approx. 2 days after maturation. Thus, while desmonemes and isorhizas start to migrate and to be mounted immediately after maturation (with a certain delay that includes the time to find the place, correct the position and accomplish cell physiological changes), the situation for stenoteles is different. The delay of 1 to 1.5 days before stenoteles start to migrate could reflect an additional maturation phase for these more complex types of nematocytes or represent the ‘reservoir’ of mature stenoteles reported by Zumstein (1973). Further experiments with Hydra that are devoid of a considerable amount of stenoteles might clarify this point. Outlook Studies of nematocyte movement and turnover, as e.g. shown in Fig. 6, can now be performed without time-consuming autoradiographic procedures in quick and straightforward experiments. It will be possible after all, in conjunction with the various well-established grafting techniques, to investigate in detail the regulation of nematocyte growth and supply, which are both obscure (Bode, 1988). In particular, assays that allow the detection, quantification and eventually characterisation of stimulating, inhibitory and chemotactic factors influencing the differentiation, migration and mounting of the different types of nematocytes can be established. In addition, TRITC-labelled nematocytes can be recorded with time-lapse video under fluorescence illumination for several hours without considerable photo-bleaching; the direct observation and analysis of migration of nematocytes in vivo (Campbell and Marcum, 1980) will thus be simplified considerably. Fur- 411 thermore, the analysis of the maturation of Hydra nematocytes, in particular the synthesis of the pressure-generating poly(γglutamic acid)s (Weber, 1989, 1990, 1994) and more detailed quantitative studies on the turnover and mounting of nematocytes will be facilitated. The method of TRITC labelling seems to be more generally applicable. We have observed that in marine cnidarians (polyps and medusae of Podocoryne, ephyra of Aurelia, acontia of Calliactis) some, presumably immature, populations of nematocytes/nematoblasts are preferentially stained and that mature fluorescent nematocytes are found mounted later. In Hydra as well as in the few other well-investigated hydrozoans it will be of interest to study the turnover and migration of nematocytes in relation to different recently discovered or postulated morphogenic factors (Fujisawa, 1987; Hassel and Berking, 1988; Müller, 1989; Plickert, 1989; Lange et al., 1990; Lange and Müller, 1991). I thank Dr Pierre Tardent for his help and everlasting generous support, Dr Marianne Klug and Dr Charles N. David for stimulating discussions, and Dr Robert Stidwill for commenting on the manuscript. The work was supported financially by the Swiss National Science Foundation (grant 31-29860.90). REFERENCES Blanquet, R. S. (1977). Cnidarian venoms. In Perspectives in Toxinology (ed. A. W. Bernheimer), pp. 149-167. John Wiley & Sons, New York. Blanquet, R. S. and Lenhoff, H. M. (1966). A disulfide-linked collagenous protein of nematocyst capsules. Science 154, 152-153. Bode, H., Berking, S., David, C. N., Gierer, A., Schaller, H. and Trenkner, E. (1973). Quantitative analysis of cell types during growth and morphogenesis in Hydra. Roux’s Arch. Dev. Biol. 171, 269-285. Bode, H. R. and Flick, K. M. (1976). Distribution and dynamics of nematocyte populations in Hydra attenuata. J. Cell Sci. 21, 15-34. Bode, H. (1988). Control of nematocyte differentiation in Hydra. In The Biology of Nematocysts (ed. D. A. Hessinger and H. M. Lenhoff), pp. 123142. Academic Press, San Diego. Bosch, T. C. G. and David, C. N. (1991). Decision making in interstitial stem cells of Hydra. In Vivo (Athen) 5, 515-520. Burnett, J. W. and Calton, G. J. (1977). Review article: The chemistry and toxicology of some venomous pelagic coelenterates. Toxicon 15, 177-196. Burnett, J. W. and Calton, G. J. (1987). Venomous pelagic coelenterates: chemistry, toxicology, immunology and treatment of their stings. Toxicon 25, 581-602. Campbell, R. D. and Marcum, B. A. (1980). Nematocyte migration in Hydra: evidence for contact guidance in vivo. J. Cell Sci. 41, 33-51. Campbell, R. D. (1988a). Migration of nematocytes in hydrozoans. In The Biology of Nematocysts (ed. D. A. Hessinger and H. M. Lenhoff), pp. 123142. Academic Press, San Diego. Campbell, R. D. (1988b). The nematocyte: an encapsulation of developmental processes. In The Biology of Nematocysts (ed. D. A. Hessinger and H. M. Lenhoff), pp. 115-121. Academic Press, San Diego. Campbell, R. D. (1989). Taxonomy of the European Hydra (Cnidaria, Hydrozoa). A re-examination of its history with emphasis on the species Hydra vulgaris Pallas, Hydra attenuata Pallas and Hydra circumcincta Schulze. Zool. J. Linn. Soc. 95, 219-244. David, C. N. (1973). A quantitative method for maceration of hydra tissue. Roux’s Arch. Dev. Biol. 171, 259-268. David, C. N. and Gierer, A. (1974). Cell cycle kinetics and development of Hydra attenuata. III. Nerve and nematocyte differentiation. J. Cell Sci. 16, 359-375. Drobnica, L., Kristian, P. and Augustin, J. (1977). The chemistry of the -NCS group. In The Chemistry of Cyanates and Their Thio Derivatives (ed. S. Patai), pp. 1003-1221. John Wiley & Sons, New York. Flick, K. M. and Bode, H. R. (1983). Dissociating tissue into cells and the development of Hydra from aggregated cells. In Hydra: Research Methods (ed. H. M. Lenhoff), pp. 251-259. Plenum Press, New York. 412 J. Weber Fujisawa, T. (1987). An endogenous inhibitor involved in position-dependent stenotele differentiation in Hydra. Dev. Biol. 122, 210-216. Gierer, A., Berking, S., Bode, H., David, C. N., Flick, K., Hansmann, G., Schaller, H. and Trenkner, E. (1972). Regeneration of Hydra from reaggregated cells. Nature 239, 98-101. Giloh, H. and Sedat, J. W. (1982). Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science 217, 1252-1255. Golz, R. and Thurm, U. (1990). Cnidocil regeneration in nematocytes of Hydra. Protoplasma 155, 95-105. Golz, R. and Thurm, U. (1991). Cytoskeleton-membrane interactions in the cnidocil complex of hydrozoan nematocytes. Cell Tiss. Res. 263, 573-583. González Agosti, C. and Stidwill, R. P. (1991). In vitro migration of Hydra nematocytes: the influence of the natural extracellular matrix (the mesoglea), of collagen type IV and type I, laminin, and fibronectin on cell attachment, migration parameters, and on patterns of cytoskeletal proteins. Cell Motil. Cytoskel. 20, 215-227. González Agosti, C. G. and Stidwill, R. P. (1992). The contributions of microtubules and F-actin to the in-vitro migratory mechanisms of Hydra nematocytes as determined by drug interference experiments. Exp. Cell Res. 200, 196-204. Greber, M. J., David, C. N. and Holstein, T. W. (1992). A quantitative method for seperation of living Hydra cells. Roux’s Arch. Dev. Biol. 201, 296-300. Günzl, H. (1971). Dipurena reesi (Hydrozoa). Wanderung der Cnidoblasten in den Rhizistolonen. In Encyclopaedia Cinematographica (ed. G. Wolf), pp. 3-15. Institut für den wissenschaftlichen Film, Göttingen, BRD. Hassel, M. and Berking, S. (1988). Nerve cell and nematocyte production in Hydra is deregulated by lithium ions. Roux’s Arch. Dev. Biol. 197, 491-495. Herlands, R. L. and Bode, H. R. (1974). Oriented migration of interstitial cells and nematocytes in Hydra attenuata. Roux’s Arch. Dev. Biol. 176, 67-88. Hessinger, D. A. and Lenhoff, H. M. (1988). The Biology of Nematocysts. Academic Press, San Diego. Holstein, T. (1981). The morphogenesis of nematocytes in Hydra and Forskalia: an ultrastructural study. J. Ultrastruct. Res. 75, 276-290. Klimek, F. (1979). Untersuchungen zur Separation und Aggregation von Hydrazellen. Ph.D. thesis, Tübingen, BRD. Kosower, N. S., Kosower, E. M., Newton, G. L. and Ranney, H. M. (1979). Bimane fluorescent labels: labeling of normal human red cells under physiological conditions. Proc. Nat. Acad. Sci. 76, 3382-3386. Kurz, E. M., Holstein, T. W., Petri, B. M., Engel, J. and David, C. N. (1991). Mini-collagens in Hydra nematocysts. J. Cell Biol. 115, 1159-1169. Lange, R. G., Holzenburg, P. and Müller, W. A. (1990). Pulses of ammonia and methylamine induce down-regulation of nematocyte and nerve cell populations in Hydrozoa (Hydra; Hydractinia). Roux’s Arch. Dev. Biol. 199, 123-133. Lange, R. G. and Müller, W. A. (1991). SIF, a novel morphogenic inducer in Hydrozoa. Dev. Biol. 147, 121-132. Loomis, W. F. and Lenhoff, H. M. (1956). Growth and sexual differentiation of Hydra mass culture. J. Exp. Zool. 132, 555-574. Mariscal, R. N. (1974). Nematocysts. In Coelenterate Biology (ed. L. Muscatine and H. M. Lenhoff), pp. 129-178. Academic Press, New York. Mariscal, R. N. (1984). Cnidaria: Cnidae. In Biology of the Integument, vol. 1, Invertebrates (ed. J. Bereiter-Hahn, A. G. Matoltsy and K. S. Richards), pp. 57-68. Springer Verlag, Berlin. Maurer, A. (1988). Investigations about the localization of the head activator in Hydra attenuata Pall. (Hydrozoa, Cnidaria). Ph.D. thesis, University of Zurich, Switzerland. Müller, A. W. (1989). Diacylglycerol-induced multihead formation in Hydra. Development 105, 309-316. Plickert, G. (1989). Proportion-altering factor (PAF) stimulates nerve cell formation in Hydractinia echinata. Cell Differ. Dev. 26, 19-28. Schmid, V., Stidwill, R., Bally, A., Marcum, B. and Tardent, P. (1981). Heat dissociation and maceration of marine Cnidaria. Roux’s Arch. Dev. Biol. 190, 143-149. Slautterback, D., B. and Fawcett, D. W. (1959). The development of the cnidoblast in Hydra. An electron microscope study of cell differentiation. J. Biophys. Biochem. Cytol. 5, 441-452. Stidwill, R. P. and Honegger, T. G. (1989). A single layer of microtubules is part of a complex cytoskeleton in mature nematocytes of Hydra. Tissue & Cell 21, 179-188. Tardent, P. (1966). Zur Sexualbiologie von Hydra attenuata Pall. Rev. Suisse Zool. 73, 357-381. Tardent, P. and Morgenthaler, U. (1966). Autoradiographische Untersuchungen zum Problem der Zellwanderungen bei Hydra attenuata Pall. Rev. Suisse Zool. 78, 468-480. Tardent, P., Holstein, T., Weber, J. and Klug, M. (1985). The morphodynamics and actions of stenotele nematocysts in Hydra. Arch. Sci. Genève 38, 401-418. Watson, M. G. and Hessinger, D. A. (1989). Cnidocyte mechanoreceptors are tuned to the movements of swimming prey by chemoreceptors. Science 243, 1589-1591. Weber, G., Honegger, T. and Tardent, P. (1978). Neuorientierung der Nesselzellwanderung bei Hydra attenuata Pall. durch transplantierte Tentakel. Rev. Suisse Zool. 85, 768-774. Weber, J., Klug, M. and Tardent, P. (1987). Some physical and chemical properties of purified nematocysts of Hydra attenuata Pall (Hydrozoa, Cnidaria). Comp. Biochem. Physiol. 88B, 855-862. Weber, J., Klug, M. and Tardent, P. (1988). Chemistry of hydra nematocysts. In The Biology of Nematocysts (ed. D. A. Hessinger and H. M. Lenhoff), pp. 427-444. Academic Press, San Diego. Weber, J. (1989). Nematocysts (stinging capsules of Cnidaria) as Donnanpotential-dominated osmotic systems. Eur. J. Biochem. 184, 465-476. Weber, J. (1990). Poly(γ-glutamic acid)s are the major constituents of nematocysts in Hydra (Hydrozoa, Cnidaria). J. Biol. Chem. 265, 9664-9669. Weber, J. (1994). The metabolism of poly(γ-glutamic acid)s of nematocysts in Hydra vulgaris: detection of two distinct hydrolytic enzymes in endoderm and in nematocysts. Comp. Biochem. Physiol. 107B, 21-32. Williams, R. B., Cornelius, P. F. S., Hughes, R. G. and Robson, E. A. (1991). Coelenterate Biology: Recent research on Cnidaria and Ctenophora. Proceedings of the Fifth International Conference on Coelenterate Biology, 1989. In Developments in Hydrobiology, vol. 66 (ed. H. J. Dumont), pp. 0742. Kluwer Academic Publ., Dordrecht, NE. Yamamoto, K., Sekine, T. and Kanaoka, Y. (1977). Fluorescent thiol reagents XII. Fluorescent tracer method for protein SH groups using N-(7dimethylamino-4-methyl coumarinyl) maleimide. An application to the proteins separated by SDS-polyacrylamide gel electrophoresis. Anal. Biochem. 79, 83-94. Zumstein, A. (1973). Regulation der Nematocyten-Produktion bei Hydra attenuata Pall. Roux’s Arch. Dev. Biol. 173, 294-318. (Received 28 March 1994 - Accepted 24 August 1994)