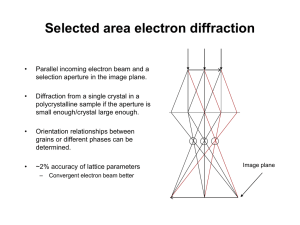
Crystallography 10
advertisement

Diffraction: Intensity
(From Chapter 4 of Textbook 2 and Chapter 9 of Textbook 1)
Electron atoms group of atoms or structure
Crystal (poly or single)
Scattering by an electron:
P
r
0
I I0
4
= /2
e4 2
K 2
2 2 sin I 0 2 sin
r
m r
by J.J. Thomson
0: 410-7 mkgC-2
2
a single electron charge e (C), mass m (kg),
distance r (meters)
z
E 2 E y2 Ez2
P
r
2
x
y component
z component
O
Random
polarized
y
1 2
1
2
2
E E y Ez I 0 y I 0 z I 0
2
2
= yOP = /2
= zOP = /2 -2
K
r2
K
I 0 z 2 cos2 2
r
I Py I 0 y
I Pz
2
K
K
K
1
cos
2
2
I P I Py I Pz I 0 y 2 I 0 z 2 cos 2 I 0 2
r
r
r
2
Polarization factor
Polarization Factor
1.0
0.8
0.6
0.4
0
20
40
60
80
100 120 140 160 180
2 (Degrees)
Pass through a monochromator first (Bragg angle M)
the polarization factor is ?
z
P
2M
r
z
Random
polarized
O
P
P
r
2
y
O
polarization is
not complete
random
anymore
y
x
x
I P I Py I Pz I 0 y
2
K
K
K
1
cos
2 M
2
I 0 z 2 cos 2 M I 0 2
2
r
r
r
2
1 cos2 2 cos2 2 M
1 cos2 2 M
(Homework)
1.0
Si (111) as monochromator
o
Cu K; M= 28.44
Polarization Factor
0.9
0.8
0.7
0.6
0.5
0.4
0
20
40
60
80
100 120 140 160 180
2 (Degrees)
Atomic scattering (or form) factor
a single free electron atoms
atomic scattering factor
amplitude scattered by atom
amplitude scattered by a single electron
x2
path different (O and dV):
R-(x1 + x2).
x1 r s0 ; x2 R r s
x1 dV
O r
s0
2
s
R
Differential atomic scattering factor (df) :
1
df
( r )e( 2i / )[ r( ss0 )]dV
Ee
Ee: the magnitude of the wave
from a bound electron
Electron density
Phase difference
Spherical integration dV = dr(rd) (rsind)
rsind
r: 0 -
:0-
: 0 - 2
dr
rsin(+d)d
d
r
d
http://pleasemakeanote.blogspot.tw/2010/02/9-derivation-ofcontinuity-equation-in.html
2
r
2
dr
(
rd
)(
r
sin
d
)
2
r
dr sin d
0 0
4 3
r
3
3
r
3
2 r dr sin d
2
r
0
r
0
r
0
=2
2r sin ddr
2
Evaluate (S - S0)r = | S - S0||r|cos
|(S - S0)|/2 = sin.
S
S0
S-S0
(S S0 ) r 2r sin cos
2
1
f ( ) df ( r )e( 2i / )[ r( ss0 )]dV
Ee
1 r
4ri sin cos /
2
f ( ) df
(
r
)
e
2
r
sin ddr
r
0
0
Ee
d cos
Let k 4 sin /
1 r
2
kri cos
f ( )
(
r
)
2
r
dr
e
d cos
r
0
0
Ee
ikr cos cos
e
eikr e ikr 2 sin kr
ikr cos 0
ikr
kr
1
f ( )
Ee
r
r 0
sin kr
4r ( r )
dr
kr
2
For n electrons in an atom
1
f ( )
Ee
n electrons
r
r 0
sin kr
4r ( r )
dr
kr
2
Tabulated
For = 0, only k = 0 sinkr/kr = 1.
1
f ( 0)
Ee
n electrons
r
r 0
4r 2 ( r )dr Z
equal to 1 bound
electrons
Number of electrons
in the atom
Anomalous Scattering:
Previous derivation: free electrons!
Electrons around an atom: free?
2
k
free electron
harmonic oscillator
d x
m 2 F kx
dt
Assume x ei0t
m
1/ 2
(
k
/
m
)
m(i0 ) e ke m k
0
Resonance frequency
2
d x
m 2 kx F (t )
Forced oscillator
dt
Assume F (t ) F0 e it
2
k
m
0
Assume x Ceit
i 0 t
2
Cm(i ) e
2
it
i0t
it
2
0
kCe F0 e
it
Cm 2 kC F0
F0
Cm m C F0 mC ( ) F0 C
m(02 2 )
2
2
0
2
0
2
x Ce
i t
x
F0
i t
e
m(02 2 )
Same frequency as F(t), amplitude(, 0)
= 0 C is ; in reality friction term exist no
Oscillator with damping (friction v)
d 2x
dx
m 2 c kx F (t ) F0eit
assume c = m
dt
dt
d 2x
dx
it
2
Assume
x
=
x
e
0
m 2 m
m0 x F
dt
dt
(i)2 x0 ix0 m02 x0 F0 / m
x0
F0
m( i )
2
0
2
Real part and imaginary part
if 0
1
2
(02 2 i )
E
0
Resonance
: X-ray frequency; 0: bounded electrons around atoms
0 electron escape # of electrons around an atom
f (f correction term)
imaginary part correction: f (linear absorption coefficient)
f +f + if
real
imaginary
Examples:
Si, 400 diffraction peak, with Cu K (0.1542 nm)
d 400 0.54309 / 4 0.13577 nm
2d 400 sin 0.1542 34.6
sin
1
0.368
sin
0.3
0.4
8.22 7.20
8.22 7.20
0.3 0.4
f 7.526
8.22 f
0.3 0.368
Anomalous Scattering correction f 0.2; f 0.4
Atomic scattering factor in this case:
7.526-0.2+0.4i = 7.326+0.4i
f and f: International Table for X-ray Crystallography V.III
Structure factor
atoms unit cell
How is the diffraction peaks (hkl) of a structure named? Unit cell
How is an atom located in a unit cell affect the h00 diffraction peak?
Miller indices (h00):
d h 00 a / h AC
3
1
A
2
1
S B R
2
C
plane a
(h00)
path difference:11 and 22 (NCM)
2211 NCM 2d h 00 sin
why:? Meaningful!
path difference: 11 and 33 (SBR)
AB
x
hx
3311 SBR
AC
a/h
a
N
3
x̂
M
phase difference (11 and 33)
3311
2 hx
2hx
a
a
position of atom B: fractional coordinate of a: u x/a.
2hx
3311
2hu
a
the same argument B: x, y, z x/a, y/b, z/c u, v, w
Diffraction from (hkl) plane
2 (hu kv lw)
amplitude scattered by all atoms of a unit cell
F
amplitude scattered by a single electron
F: amplitude of the resultant wave in terms of the amplitude
of the wave scattered by a single electron.
N atoms in a unit cell; fn: atomic form factor of atom n
F f1e 2i ( hu1 kv1 lw1 ) f 2 e 2i ( hu2 kv2 lw2 ) f N e 2i ( hu N kvN lwN )
N
Fhkl f n e2i ( huN kv N lwN )
n 1
F (in general) a complex number.
How to choose the groups of atoms to represent a unit cell
of a structure?
1. number of atoms in the unit cell
2. choose the representative atoms for a cell properly (ranks of
equipoints).
Example 1: Simple cubic
1 atoms/unit cell;
000 and 100, 010, 001, 110, 101, 011, 111: equipoints of rank 1;
Choose any one will have the same result!
Fhkl fe 2i ( h 0 k 0l 0 ) f
Fhkl f
2
2
for all hkl
Example 2: Body centered cubic
2 atoms/unit cell;
000 and 100, 010, 001, 110, 101, 011, 111: equipoints of rank 1;
½ ½ ½: equipoints of rank 1;
Two points to choose: 000 and ½ ½ ½.
1 1 1
2i ( h k l )
2 2 2
Fhkl fe 2i ( h 0 k 0l 0 ) fe
Fhkl 2 f when h+k+l is even
Fhkl 0
when h+k+l is odd
f (1 ei ( h k l ) )
Fhkl 4 f
2
Fhkl 0
2
2
Example 3: Face centered cubic
4 atoms/unit cell;
000 and 100, 010, 001, 110, 101, 011, 111: equipoints of rank 1;
½ ½ 0, ½ 0 ½, 0 ½ ½, ½ ½ 1, ½ 1 ½, 1 ½ ½: : equipoints of rank 3;
Four atoms chosen: 000, ½ ½ 0, ½ 0 ½, 0 ½ ½.
Fhkl fe 2i ( h 0 k 0l 0 ) fe
1 1
2i ( h k l 0 )
2 2
fe
1
1
2i ( h k 0 l )
2
2
fe
1 1
2i ( h 0 k l )
2 2
f [1 ei ( h k ) ei ( k l ) ei ( h l ) ]
Fhkl 4 f
when h, k, l is unmixed (all evens or all odds)
Fhkl 16 f
2
Fhkl 0
2
when h, k, l is mixed
Fhkl 0
2
Example 4: Diamond Cubic
8 atoms/unit cell;
000 and 100, 010, 001, 110, 101, 011, 111: equipoints of rank 1;
½ ½ 0, ½ 0 ½, 0 ½ ½, ½ ½ 1, ½ 1 ½, 1 ½ ½: equipoints of rank 3;
¼ ¼ ¼, ¾ ¾ ¼, ¾ ¼ ¾, ¼ ¾ ¾: equipoints of rank 4;
Eight atoms chosen: 000, ½ ½ 0, ½ 0 ½, 0 ½ ½ (the same as FCC),
¼ ¼ ¼, ¾ ¾ ¼, ¾ ¼ ¾, ¼ ¾ ¾!
Fhkl fe 2i ( h 0 k 0l 0 ) fe
fe
Fhkl
1 1 1
2i ( h k l )
4 4 4
1 1
2i ( h k l 0 )
2 2
fe
fe
3 3 1
2i ( h k l )
4 4 4
1
1
2i ( h k 0 l )
2
2
fe
fe
3 1 3
2i ( h k l )
4 4 4
1 1
2i ( h 0 k l )
2 2
fe
1 3 3
2i ( h k l )
4 4 4
1 1
1
1
1 1
2i ( h k l 0 )
2i ( h k 0 l )
2i ( h 0 k l )
2
2 2
f 1 e 2 2 e 2
e
1 1 1
2i ( h k l )
4 4 4
1
e
FCC structure factor
Fhkl 4 f (1 i ) when h, k, l are all odd
Fhkl 8 f
Fhkl 32 f
when h, k, l are all even and h + k + l = 4n
Fhkl 64 f
2
2
Fhkl 4 f (1 1) 0 when h, k, l are all even and
2
h + k + l 4n
Fhkl 0
2
Fhkl 0 when h, k, l are mixed
Fhkl 0
2
2
Example 5: HCP
2 atoms/unit cell
8 corner atoms: equipoints of rank 1;
1/3 2/3 ½: equipoints of rank 1;
Choose 000, 1/3 2/3 1/2.
Fhkl fe 2i ( h 0 k 0l 0 ) fe
1 2 1
2i ( h k l )
3 3 2
Set [h + 2k]/3+ l/2 = g
(001) ( 1/3 2/3 1/2)
(000)
(010)
(100) (110)
equipoints
Fhkl f (1 e 2ig )
Fhkl f 2 (1 e 2ig )(1 e 2ig ) f 2 (2 2 cos 2g ) 4 f 2 cos 2 g
2
Fhkl
2
h 2k l
4 f cos g 4 f cos (
)
3
2
2
2
2
2
h + 2k
l
h 2k l
cos (
)
3
2
3m
3m
3m1
3m1
even
odd
even
odd
1
0
0.25
0.75
2
Fhkl
4f 2
0
f2
3f 2
2
Multiplicity Factor
Equal d-spacings equal B
E.g.: Cubic
(100), (010), (001), (-100), (0-10), (00-1): Equivalent
Multiplicity Factor = 6
(110), (-110), (1-10), (-1-10), (101), (-101), (10-1),(-10-1),
(011), (0-11), (01-1), (0-1-1): Equivalent
Multiplicity Factor = 12
lower symmetry systems multiplicities .
E.g.: tetragonal
(100) equivalent: (010), (-100), and (0-10)
not with the (001) and the (00-1).
{100} Multiplicity Factor = 4
{001} Multiplicity Factor = 2
Multiplicity p is the one counted in the point group
stereogram.
In cubic (h k l)
{hkl} p = 48 3x2x23 = 48
{hhl} p = 24 3x23 = 24
{0kl} p = 24 3x23 = 24
{0kk} p = 12 3x22 = 12
23 = 8
{hhh} p = 8
3x2 = 6
{h00} p = 6
Lorentz factor:
dependence of the integrated peak intensities
1. finite spreading of the intensity peak
1
sin 2
2. fraction of crystal contributing to a diffraction peak cos
1
3. intensity spreading in a cone
sin 2
2
1
Imax
B
1
2
2
Intensity
1 B
2 B
path difference for 11-22
= AD – CB = acos2 - acos1
= a[cos(B-) - cos (B+)]
= 2asin()sinB ~ 2a sinB.
2Na sinB = completely
cancellation (1- N/2, 2- (N/2+1) …)
1
Imax/2
B
Integrated
Intensity
2B
Diffraction Angle 2
1
2
2
D
C
2
a
A
1
B
N
Na
Maximum angular range of the peak
2 Na sin B
Imax 1/sinB,
Half maximum B 1/cosB (will be shown later)
integrated intensity ImaxB (1/sinB)(1/cosB) 1/sin2B.
2
number of crystals orientated at or near the Bragg angle
N 2r sin(90 B ) r
r sin( 90 B )
/2-
Fraction of crystal:
N 2r sin(90 B ) r
2
N
4r
cos B
2
crystal plane
r
3
diffracted energy:
equally distributed (2Rsin2B)
the relative intensity per unit length 1/sin2B.
2B
Lorentz factor:
1
1
cos
cos B
Lorentz factor
2
sin
2
sin
2
sin
2 B
B
B
1
4 sin 2 cos
Lorentz–polarization factor:
(omitting constant)
1 cos2 2
Lorentz - polarization factor 2
sin cos
Lorentz-Polarization Factor
100
80
60
40
20
0
0
20
40
60
80 100 120 140 160 180 200
2 (Degrees)
Absorption factor:
X-ray absorbed during its in and out of the sample.
Hull/Debye-Scherrer Camera: A(); A() as .
Diffractometer:
Incident beam: I0; 1cm2
incident angle .
Beam incident on the plate: I 0 e ( AB )
a: volume fraction of the specimen that
are at the right angle for diffraction
b: diffracted intensity/unit volume
I0 1cm dID
C
x A
B
dx
l
2
: linear absorption
coefficient
volume = l dx 1cm = ldx.
actual diffracted volume = aldx
Diffracted intensity: ablI 0 e ( AB) dx
Diffracted beam escaping from the sample: ablI 0 e ( AB ) e ( BC ) dx
1
x
x
l
; AB
; BC
sin
sin
sin
I 0 ab
dI D
e
sin
If = =
ID
x
x 0
1
1
x
sin sin
dx
I 0 ab 2 x / sin
dI D
e
dx
sin
2 x
I 0 ab x sin 2 x I 0 ab
dI D
e
d
x
0
2
sin 2
Infinite thickness ~ dID(x = 0)/dID(x = t) = 1000 and = = ).
Temperature factor (Debye Waller factor):
Atoms in lattice vibrate (Debye model)
(1) lattice constants 2 ;
Temperature (2) Intensity of diffracted lines ;
(3) Intensity of the background scattering .
u
u
d
low B
d
high B
Lattice vibration is more significant at high B
(u/d) as B
Formally, the factor is included in f as f f 0 e M
Because F = |f 2| factor e-2M shows up
What is M?
2
2
2
u
2 sin B
sin B
M 2 2 2 2 2 u 2
B
d
u 2 : Mean square displacement
Debye: M
2
6h T
mk 2
x sin B
(
x
)
4
2
h: Plank’s constant;
T: absolute temperature;
m: mass of vibrating atom;
: Debye temperature of the substance; x = /T;
(x): tabulated function
u 0
u
u2 0
e-2M
6h 2T 1.15 10 4 T
m atomic weight (A):
2
mk
A 2
1
0
sin /
I
TDS
2 or sin/
Temperature (Thermal) diffuse scattering (TDS) as
I as
peak width B slightly as T
Summary
Intensities of diffraction peaks from polycrystalline samples:
Diffractometer:
2
1
cos
2 2 M
2
2
e
I N F p 2
sin cos
Other diffraction methods:
2
1
cos
2
2
A( )e 2 M
I N F p 2
sin cos
2
Match calculation? Exactly: difficult; qualitatively matched.
Perturbation: preferred orientation; Extinction (large crystal)
Example
Debye-Scherrer powder pattern of Cu made with
Cu radiation
Cu: Fm-3m, a = 3.615 Å
1
line
1
2
3
4
5
6
7
8
2
3
hkl h2+k2+l2
111
3
200
4
220
8
311
11
222
12
400
16
331
19
420
20
4
sin2
0.1365
0.1820
0.364
0.500
0.546
0.728
0.865
0.910
5
sin
0.369
0.427
0.603
0.707
0.739
0.853
0.930
0.954
6
7
(o) sin/(Å-1)
21.7
0.24
25.3
0.27
37.1
0.39
45.0
0.46
47.6
0.48
58.5
0.55
68.4
0.60
72.6
0.62
8
fCu
22.1
20.9
16.8
14.8
14.2
12.5
11.5
11.1
Structure Factor
F 4 f Cu
F 0
111
200
220
311
222
400
331
420
If h, k, l are unmixed
If h, k, l are mixed
1
9
10
line
|F|2
P
1
2
3
4
5
6
7
8
7810
6990
4520
3500
3230
2500
2120
1970
8
6
12
24
8
6
24
24
11
12
13
14
1 cos 2 2 Relative integrated intensity
sin 2 cos Calc.(x105) Calc.
Obs.
12.03
7.52
10.0
Vs
8.50
3.56
4.7
S
3.70
2.01
2.7
s
2.83
2.38
3.2
s
2.74
0.71
0.9
m
3.18
0.48
0.6
w
4.81
2.45
3.3
s
6.15
2.91
3.9
s
1
h2 k 2 l 2
2
2
d hkl
a
a
d111
3
3.615
2d111 sin 111 1.542 2
sin 111 1.542
3
sin 111 0.3694 111 21.68o
sin 111
sin
0
0.3694
0.24
1.542
0.1
0.2
0.3
0.4
29 27.19 23.63 19.90 16.48
0.3 0.24
0.3 0.2
111
f
Cu 22.1
111
19.90 f Cu
19.90 23.63
111 2
F111 ( 4 f Cu
) 7814
2
{111}
p=8
(23 = 8)
1 cos 2111
12.05
2
sin 111 cos 111
2
2
1 cos 2
2
2
2 M
I N F p 2
A( )e
sin cos
I 753370
Dynamic Theory for Single crystal
Kinematical theory
Dynamical theory
S0
Refraction
PRIMARY EXTINCTION
K0
S
K0
K1
K1
K2
K2
K0 & K1 : /2; K1 & K2 : /2
K0 & K2 : ; destructive interference
K1 K2
(hkl)
Negligible absorption
8
I
3
e 2 N2 | F | 1 | cos 2 |
2
2
mc sin 2
I |F| not |F|2!
e: electron charge; m: electron mass; N: # of unit cell/unit volume.
Width of the diffraction peak (~ 2s)
e 2 N2 | F | 1 | cos 2 |
s 2
2
mc sin 2
FWHM for Darwin
curve = 2.12s
5 arcs < < 20 arcs