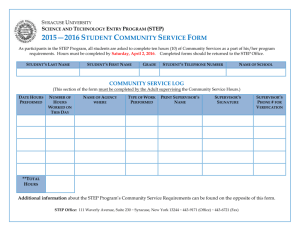
aspects of this instructional cycle
advertisement

ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu A Preliminary Examination of a New Instructional Model for Conceptual Change. GLENN DOLPHIN Department of Science Teaching Syracuse University Syracuse, NY grdolphi@syr.edu Abstract: This paper is the result of a pilot study of the efficacy of a new instructional model for conceptual change. The instructional model blends aspects from typically separate schools of thought, including model based learning, writing to learn and drawing to learn, and features of the nature of science. Science notebooks play a central role in documenting students’ creation of knowledge and evolution of understanding. Instructors implemented this new instructional approach in an introductory physical science course to teach the particulate nature of matter to undergraduate preservice elementary teachers. The instructional model prompts students to elicit their prior knowledge, through writing and diagramming, write testable questions, develop predictions and procedures for inquiries, and conduct experiments. They collect and analyze their own data and fashion claims based on gathered evidence. Through reflective activities, small group and whole class discussions, students relate their claims back to their original mental model to check for congruency, and describe how their original model was either supported or refuted by their new information. Summative assessment data based on open-ended exam questions and an essay assignment provide some evidence of student metacognitive processing and conceptual content change. Many students recognized that their initial understanding of matter was very general and did not incorporate descriptions of particles. Some students even recognized that the very act of writing the essay helped them to deepen their understanding and build their mental model. The paper outlines this integrated instructional model, describes some of the evidence supporting the claim of deepening understanding, discusses some implications for teaching the nature of science, and proposes areas of future possible research. 1.0 Introduction The last 30 years has seen a shift in science education in our understanding of how students learn. Students in science classrooms are no longer seen as “empty vessels” waiting to be filled, or “blank slates” waiting to be written on. It has become increasingly obvious that students come to school, even in the earliest years, with an understanding of how the world works based on their own observations and reasoning abilities. These understandings or conceptions come in various levels of completeness and accuracy compared to current scientific thought. Researchers in science education have learned that these “naïve,” “alternative,” or 1 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu “mis-” conceptions are not only tenacious, but will fundamentally impact what and how the student will learn. In this light, researchers have created and tested different instructional models, for instance, the 5-E cycle (Bybee, Taylor, Gardner, Van Scotter, Bloom, Moran et al., 2006), model co-construction and evolution (Khan, 2008), Gagné’s nine events of instruction (Gagné, Briggs & Wager, 1992), and mediated modeling (Halloun, 2007, 2004). However, these methods of instruction are founded on what Bereiter (2002) calls a folk theory of mind. This theory considers the mind a container and knowledge something that is put into and/or modified within the container. He goes on to claim that this theory of mind is losing its usefulness in a society where knowledge creation is becoming more important. “A viable theory of mind for 21st century education, it seems to me, must be able to negotiate effectively between individual learning on the one hand and knowledge conceived of as a product or as a cultural good on the other” (p. 20). He sees the container metaphor as being adequate for explaining how learners acquire knowledge but insufficient to explain what that knowledge can be used for once it is there. Sfard (1998) also has misgivings for this model of learning - what she refers to as the acquisition metaphor. This metaphor also posits the mind as a vessel for knowledge acquisition. She points out that according to the acquisition metaphor, the learner must rely on previous knowledge in order to acquire new knowledge. This brings up the paradox: How can the learner rely on the previous knowledge of something that (s)he does not already know? In contrast to the acquisition metaphor, Sfard describes a second model of learning - the participation metaphor. Here, knowledge is not separate and objectified but part of the interaction between the learner and his or her environment. Instead of gathering information as one would possessions, the participation metaphor explains learning as happening during discourse and 2 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu practice situated within the context of a community. Bereiter (2002) acknowledges that all human behavior is situated, however he also points out that school mathematics, for example “is not knowledge that is embedded in a community of practice but rather is knowledge there for the taking, by anyone who has access to it and who can make something of it” (p.61). Due to the differences between each metaphor in how they define knowledge, learning, and understanding, they each prescribe different strategies of instruction. The acquisition metaphor advocates such strategies as activating prior knowledge, advance organizers (concept maps, outlines, Venn diagrams, etc) mental modeling and diagramming. The participation metaphor advocates learning communities and cooperative/collaborative groups, rehearsal, and cognitive apprenticeships and coaching. Even though many researchers find themselves defending one or the other metaphor as being the closest to explaining how learning actually takes place (see Anderson, Redder, & Simon, 1996; Anderson, Redder, & Simon, 1997; Greeno, 1997 for an example of this), Sfard (1998) states that not only are both metaphors useful, but they should be used together so that one can fill in the gaps the other one leaves. She goes on to state that the two metaphors are not mutually exclusive but incommensurable. “Thus, for instance, today’s mathematicians are able to live with Euclidean and non-Euclidean geometries without privileging any one of them, whereas contemporary physicists admit a mixture of ostensibly contradictory approaches to subatomic phenomena, sanctioning this decision with Bohr’s famous principle of complementarity” (p. 11). 2.0 Toward a New “Inclusive” Theoretical Framework 3 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu Paavola and Hakkarainen (2005) acknowledge the contributions of the theories supporting the acquisition and participation metaphors. However, they also point out a deficiency in both. Where the acquisition metaphor focuses on the delivery of knowledge to the mind and the participation metaphor focuses on the transference of knowledge from person to person, neither one makes accommodation for the generation of new knowledge. Paavola et al. (2004) cite three knowledge creation models, Nonaka and Takeuchi’s (1995) model of knowledge creation, Engeström’s (1999) model of expansive learning, and Bereiter’s (2002) model of knowledge building, to draw attention to the significant gap in the acquisition and participation metaphors; neither adequately address the creation of new knowledge. With the motivation to emphasize the importance of the acquisition and participation metaphors, and fill this gap, Paavola et al. (2004) propose a third metaphor of learning - the knowledge creation metaphor. They describe it as model that “conceptualize[s] learning and knowledge advancement as collaborative processes for developing shared objects of activity. Learning is not conceptualized as occurring in the individuals’ minds, or through processes of social practices. Learning is understood as a collaborative effort directed toward developing some mediated artifacts, broadly defined as including knowledge, ideas, practices and material or conceptual artifacts” (pp. 569-570). Within the context of the knowledge creation metaphor, Paavola and Hakkarainen (2005) call for a change in the educational system. They argue that schools should become communities where teachers and students alike have rich experiences in knowledge building. They emphasize a model of progressive inquiry where “explanation seeking processes and collaborative and social aspects of learning are emphasized. The model depicts a deepening question-explanation process where students collaboratively create context, set up research questions, construct 4 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu working theories and critically evaluate the process” (p. 549). The knowledge creation metaphor differs from the acquisition metaphor in that it does not focus on the transfer of knowledge but rather the creation of new knowledge. However, the individual is still important as the bringer of knowledge and intuition that can be used to solve problems posed to the group. Although the work of knowledge creation does happen within a social context, as it does in the participation metaphor, it differs from that model because the focus is not on the dynamics of group interaction but on the product of that interaction (Paavola & Hakkarainen, 2005). They encourage the use of all the metaphors to capture the learning process best. 3.0 The Unifying Instructional Model The instructional model proposed in this paper is situated within the framework of the knowledge creation metaphor (Paavola & Hakkarainen, 2005; Paavola et al., 2004). Within this framework, “explanation seeking processes, and collaborative and social aspects of learning are emphasized…the most promising tools are ones that guide the participants themselves to engage in extensive working to produce knowledge through writing and visualization.” (p. 549). Paavola and Hakkarainen (2005) also encourage students to make notes, pose questions, explain their own understandings and finally present knowledge gains thereby “organizing the learning community’s activity around shared objects of inquiry” (p. 550). Essentially, the knowledge creation metaphor incorporates the cognitive sciences with socio-constructivist epistemologies and then adds a new facet. This facet emphasizes the importance of mediating artifacts. Mediating artifacts can be concepts, theories, activities or practices. Instruction is successful when students are working in small groups, constructing knowledge in the form of these artifacts, individually and as a group, and revisiting previous understandings in light of new 5 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu observations. Bereiter (2002) encourages telling students that they are not developing a relationship with the actual phenomenon but rather with their understanding of the phenomenon being investigated. Paavola and Hakkarainen (2005) argue that although young children may not be in a position to create knowledge in a historic sense, they should be creating knowledge relative to their original position. The general progression of the new instructional cycle has been modeled after the “levels of target concepts,” as described by Rae-Remirez (2008). Within this structure, the instructor chooses an “optimum integrated target concept” (p. 49). This can come from learning standards (AAAS, 1993; NRC, 1996) and is a major theme or “big idea” to be taught. For instance, standard 4D “Structure of Matter” (AAAS, 1993) would be an example of such an optimum integrated target concept. The instructor parses this major theme out into several different target models (target model 1 + target model 2 + target model 3 = optimum integrated target concept). This would correspond to “Atoms and Molecules,” “Conservation of Matter,” “States of Matter,” and “Chemical Reactions” (AAAS, 2001). The instructor then plans several instructional cycles within the context of each target model to lead students though a gradual enrichment or sophistication of intermediate models until the appropriate target model has been built (intermediate model 1a → intermediate model 1b → intermediate model 1c → target model 1). Khan (2008) includes the following indicators for recognizing an enriched intermediate model. Indicators that a student’s expressed model appears to be more enriched include: (a) an addition of new variables to the model, (b) an addition of modifiers to relationships within the model, (c) use of relationships within the model to explain novel lab findings, (d) change of drawings of molecular structures, and (e) 6 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu ability to make predictions and offer explanations that students were not able to do…prior to the learning episode. (p. 60-61) Each cycle (Figure 1) within the progression begins with the instructor eliciting students’ prior knowledge through a demonstration or questions about the phenomenon to be studied. The students express their mental model either through writing or drawing diagrams with spatial/static and causal/dynamic aspects. They do this with an emphasis on addressing the demonstration or answering the questions. The instructor then provokes conceptual dissatisfaction or cognitive dissonance by performing a discrepant event or asking one or more discrepant questions (Rea-Remirez & Núñez-Oviedo, 2008). The instructor directs the class into one of two possible paths. Path I revolves around a large group discussion about the concept where competing student ideas can be expressed, rationalized and argued (Núñez-Oviedo & Clement, 2008; Núñez-Oviedo, Clement, & Rea-Remirez, 2008). Utilizing multiple representations of the concept during whole class discussion provides information for helping students modify their mental model (Boulter & Buckley, 2000) and create meaning for the whole group. Results of this discussion become the subject of a reflective writing assignment, or conceptual artifact of Bereiter (2002), where students record the effect of the discussion on their mental model and compare and contrast their new model with the model they held previously. The emphasis on this and all writing is one of the future utility of this knowledge and not just for assessment. From cognitive dissonance, path II involves the instructor giving boundaries or parameters for exploration into the concept by having students work on an inquiry activity. Boundaries could be anything from the set of substances to be used in an investigation of chemical reactions to a discussion of the concept being explored. It is a way to scaffold the 7 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu investigations to support the students’ inquiry and prevent investigations from becoming a practice of “trial and error.” Students write testable questions and develop and defend predictions and procedures, based on their mental model. They conduct experiments, collect and analyze data, and fashion claims based on gathered evidence. Through reflective activities and small group and whole class discussions, students relate their claims back to their original mental model to check for congruency, and describe how their original model was either supported or refuted by their new information. Students keep all of their information in a science notebook, an artifact of the knowledge creation process, to reference their prior understandings for comparison to newer ones and for refining their mental model. The instructor initiates another cycle with a new discrepant event to push students to further refine their mental model. 4.0 Review of Instructional Strategies The instructional cycle proposed here combines many of the strategies traditionally separated between the two metaphors of learning. This section lists the strategies utilized within the instructional cycle and discusses its research based role in enhancing student understanding and knowledge creation. The strategies appear below in a certain sequence. This is merely an artifact of the linear nature of the writing process. There is really no specific order for the strategies as will be illustrated in the accompanying figures. The prime objective is to focus on “the richness of what there is to be learned. Major theories have great depth and wide implications. Coming to understand a living theory means establishing a many-faceted relationship and one that will keep developing as one’s experience grows and the theory itself evolves” (Bereiter, 2002, p 119). 4.1 Accessing prior knowledge 8 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu The instructional cycle, shown in Figure 2, begins with eliciting students’ prior knowledge and then creating dissatisfaction within the students concerning that prior understanding. The importance of prior knowledge within the scope of learning has been emphasized since the 1950’s within the framework of cognitive information processing and later in the 1970’s with meaningful learning and schema theory (Driscoll, 2005). Appleton (1997) found the importance of activating students’ prior knowledge and eliciting conceptual dissatisfaction through the use of teacher derived discrepant events. By studying middle school science students (11-13 years of age) through videotaped lessons, interviews, and field observations, Appleton (1997) gained insight into the cognitive response of research participants to constructivist teaching methodologies. His model of conceptual development begins with the notion that “[t]he learner brings to the learning situation all previous experiences and feelings…which are used to interpret and make sense of any new encounter” (p. 308). Taber (2003) describes a similar notion from his study of first and second year college chemistry students. After conducting student interviews and analyzing student writings and diagramming activities, he posits that college students’ learning about chemical bonding “was found to be strongly channeled by prior learning” (p. 751). Taber (2003) emphasizes the importance of uncovering students’ prior understandings, identifying possible misconceptions, and informing teachers of the learning process. With this information, teachers may adapt instruction to facilitate the conceptual change process. In their study of eighth grade students in a physical science class, Smith, Maclin, Grosslight, and Davis (1997) found that the beliefs students come to class with profoundly affect what they learn during instruction. In their study, they found that students did not come to have an accurate understanding of density until they first understood 9 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu that matter would continue to exist when broken up, even if the pieces were too small to be observed. In a study of introductory college physics students’ learning about special relativity, Posner, Strike, Hewson, and Gertzog (1982) found that students did not accept a new concept unless there was a need for the new concept. Their interpretation of the interviews of the physics students was that unless there was dissatisfaction with their current understandings of a concept, students were not likely to exchange their explanation with an explanation that more closely parallels current scientific understanding. This “dissatisfaction” can be brought about through the use of an anomaly, an experience that cannot be reconciled with the students’ current understanding (Posner et al., 1982). Only in the face of a need to make sense of an occurrence were students open to alternative conceptions. Posner et al. (1982) also note that the alternative explanation needs to be understandable, plausible, and fruitful. Subsequent critiques of this model (Pintrich, Marx, & Boyle, 1993; Strike & Posner, 1992) broaden the model to also include students’ attitude, motivation and self-efficacy as crucial determinants to conceptual change. 4.2 Diagramming Diagramming is an important aspect at various stages along the instructional cycle (Figure 3). It can be utilized for expressing both prior and newly acquired knowledge, making predictions, and supporting claims. In her study of 40 fifth grade students, Gobert (2000; 2005) had students read text about plate tectonics and had them draw diagrams to represent what they had just read. Students then made inferences about related geologic phenomena based on their diagrams. It is the use of the diagram, or conceptual artifact, as a tool to further one’s knowledge 10 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu that reflects the the knowledge creation metaphor. Gobert’s (2000) results highlight the efficacy of having students draw diagrams, as opposed to being given diagrams of a concept. Results from these studies suggest that young students can construct rich mental models of complex causal and dynamic systems, which they can then use to make inferences. These data also suggest that developing rich integrated causal models may be facilitated when models are constructed by the learner beginning with the static components first followed by increasingly complex models involving causal and dynamic information. (p. 965, emphasis mine) Gobert and Clement (1999) and Gobert (2005) describe the strength of student-generated models in developing strong conceptual understanding. Fifth grade students read passages describing plate tectonics. One group answered questions about the text, another group intermittently wrote summaries of the text and then answered questions about the text, and a third group intermittently drew diagrams summarizing the reading, and then answered questions. The researchers found that though the written summaries for the text were more detailed than the student generated diagrams, the students who did the diagramming tended to show better understanding for spatial, causal and dynamic information than the group who wrote summaries and the group who did nothing after the reading. 4.3 Writing With the emphasis on literacy becoming more potent, writing in science is also becoming more common. Different types of writing activities can occur along the instructional cycle as demonstrated in Figure 4. Keys (2000) and Keys et al. (1999) experimented with a Science Writing Heuristic (SWH) to see if purposeful writing during laboratory activities would help 11 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu eighth grade students build new knowledge. The SWH consists of a template of teacher designed activities and a template for student thinking (Keys et al., 1999). The teacher designed writing activities include concept mapping, writing about activities that include students’ personal meanings, data interpretation and comparison with others, individual reflections, and writings for presentation to the whole class. The student template consists of a number of guiding questions to facilitate understanding throughout the activity, such as “What did I do? What did I see? What can I claim? How do I know? How do my ideas compare with other ideas? How have my ideas changed?” (p. 1069). Keys et al. (1999) found that the use of SWH embedded within laboratory activities encouraged students to construct knowledge and generate meaning in the content domain and to a modest effect in the nature of science. Using the same SWH in a different class, Keys (2000) found that some students utilized writing specifically to reflect on the science content, solve problems, and generate new knowledge. Klein (2004) outlines four different factors that led to higher learning outcomes from a group of undergraduate non-science majors writing about a scientific activity in which they participated. The first factor was setting content goals. Students were told explicitly that the writing they were doing was for their own learning. The second factor was the student use of experimental results as a resource for idea and text generation. This illustrates the importance of utilizing a science notebook. The third factor was the use of comparison writing while describing the activity. Lastly, he describes student difficulty in writing as being an indicator of knowledge construction. The most common use for writing assignments has been as a form of assessment. However, as noted above, it is important to emphasize that the writing is not the end of, but merely a part of the learning process and what the students write should be used in furthering their understanding. 12 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu 4.4 Multiple Modes of External Representations “A model in science is a representation of a phenomenon initially produced … as a simplification of the phenomenon to be used in enquires” (Gilbert, Boulter, & Elmer, 2000, p. 11). However, many students see models (see Figure 5) as “replicas” of reality and see their purpose as mainly the transference of knowledge about the phenomenon (target) rather than tools for inference or prediction (Grosslight, Unger, Jay, & Smith, 1991). Boulter and Buckley (2000) outline and define the different modes and attributes of representations that can be used in instruction. Utilizing a variety of representations for a single concept can help students develop a more robust understanding of the target phenomenon (Boulter & Buckley, 2000; Grosslight et al., 1991). One caveat to this claim is that the purpose for each model be made explicit to students to avert possible confusion. For instance, Flodin (2009) found that several different models used for the target, “gene,” occurred within the same textbook – “gene as trait, an information structure, an actor in the cell, a regulator in embryonic development, or as marker for evolutionary change” (p. 91) – but without explicitly stating these differences or the overlapping and/or related functions. This can cause a great deal of confusion for the student. Different strategies for model use in science class have been shown to be effective. Students’ explicit revision of models and model use for solving problems has also been shown to increase their constructive epistemologies (Grosslight et al., 1991). In their study of eighth grade students, Ardac and Akaygun (2005) found that the use of dynamic models representing chemical changes at the molecular level were more effective than static models of the same targets for facilitating student understanding of that phenomenon. Else, Clement, and RaeRemirez (2008) outline several strategies for effective model use in the classroom. These 13 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu strategies include discussing student understandings in light of the model and using drawings and diagrams to map out the relationship between model and target. They also emphasize the importance of highlighting that models are representations with a specific purpose, and that certain aspects of the target are represented, and certain aspects of the target are not represented. 4.5 Socially Mediated Activities Much of the learning happening in classrooms is social in nature (see Figure 6). “Learning Science involves young people entering into a different way of thinking about and explaining the natural world; becoming socialized to a greater or lesser extent into the practices of the scientific community with its particular purposes, ways of seeing, and ways of supporting its knowledge claims” (Driver, Asoko, Leach, Mortimer, & Scott, 1994). There is learning that happens among students in small and large group discussions. There is learning that happens during interactions between teacher and student. Nunez-Oviedo and Clement (2008) describe a “competition strategy” for learning in which students present their own ideas in discussion. These ideas may be contradictory to each other and this contradiction fosters cognitive dissonance in the students as they try to figure out which idea best suits observations. During this process, the group created meaning through different modes of model evolution, including dropping criticized models out of discussion (disconfirmation mode), dropping, adding or changing aspects of particular models (modification mode), creating new models (generation mode), and agreeing on a model that fits the parameters best (confirmation mode). Piaxão, Caldo, Ferreira, Alves, and Morais (2004) demonstrated the efficacy of group discussion and reflection when teaching plate tectonics to seventh grade students. Students were given prompts in terms of a question or a hypothetical situation. They had to then determine a 14 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu response and discuss reasons for their response within groups or the whole class. The discussion technique of Piaxão et al. (2004) revolves around utilizing historical aspects of the development of the theory of plate tectonics. As such, they found that the strategy not only demonstrated how scientists (and students) learn, but also how scientific knowledge is created by a community of scientists (and a community of students). The conversations between teacher and student can be just as important in the learning process. Within their analysis of teacher talk, Ryder and Leach (2006) found that if teachers employed certain strategies during their classroom talk, they would have a positive impact on students’ epistemologies of science. These strategies included making appropriate statements about the epistemology of science, linking scientific epistemology with science content, and stating and justifying learning aims. They describe the following strategies as having a positive impact on students’ epistemologies of science: “(a) asking for students’ ideas, (b) highlighting students’ ideas during whole class discussions, (c) asking students to justify their ideas, (d) challenging students’ ideas, (e) evaluating students’ ideas, and (f) introducing known student misconceptions” (p. 305). Many of these same strategies were utilized successfully to promote the evolution of students’ mental models in a biology class (Núñez-Oviedo, Clement, & ReaRemirez, 2008). Their qualitative data of teacher-student(s) interaction demonstrated how the teacher facilitated the evolution of the students’ mental models of concepts such as “valves inside veins,” and diffusion in a biology class. Syh-Jong (2007) studied how students in a teacher preparation program taking a physical science program were able to construct knowledge within the content domain as a result of writing and talking in a small group format. The students broke into groups and performed the activities within the topic and discussed and wrote about their experiences within a journal. 15 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu Once all of the activities in the topic had been accomplished and discussed, the group made a presentation of findings to the rest of the class. If there were discrepancies within the class about findings, discussions ensued to rectify them. At the end of the course Syh-Jong noted that (a) The majority of students felt the teaching/learning experience to be effective. (b) Students coconstructed knowledge from working, speaking and writing in small groups. (c) Speaking and writing in small groups was responsible for their explicit understanding of knowledge within the content domain. (d) Small group, collaborative work encouraged the students to become active learners within the course. What he found was that most students felt that this teaching/learning environment was conducive to good learning. He also noted that students in the class transitioned from passive students to active, full participants within the small group environment. He posits that “speaking and writing in a collaborative group mutually stimulated students in constructing knowledge” (p. 78). In other words, writing helped students organize their thoughts for speaking to the rest of their group, while speaking helped them make explicit what was implicit in their writing. 4.6 Metacognitive Activities “Metacognition refers to one’s awareness of thinking and the self regulatory behavior that accompanies this awareness” (Driscoll, 2005, p. 107). Though activities that incorporate metacognition overlap and incorporate many of the previously described strategies, it is treated as a separate entity here to emphasize its importance (see Figure 7). In a study about the mental models undergraduate chemistry students hold regarding the structure of matter, FloresCamacho, Gallegos-Cázares, Garritz, and García-Franco (2007) found that the students could hold multiple mental models of matter at any one time. They also found that the multiple models 16 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu held by any one student could be incommensurable with each other, meaning that parts of each model may utilize the same term, however that term is assigned a different and incompatible meaning for each model making the models incomparable. They found this to have an impact on student learning. Implications from this study suggest the importance of making students aware of their different mental models and the context in which they are used. Students can analyze their models for limitations with the intent to get them to discard the less effective ones in favor of the ones better aligned with current scientific thought (Camacho, et al., 2007). Inquiry skills and scientific ability in biology were shown to be positively influenced by metacognitive activities in a group of tenth grade biology students (Zion, Michalsky, & Mevarech, 2005). Activities included posing problems to students and then having them write about their problem solving thinking. Students described their goals and strategies for solving the problem as well as their rationales. They assessed their own activity during the process, describing what difficulties they encountered. Zion et al. (2005) also found that students communicating in text through a computer interface with students from another school (asynchronous learning network) also had higher performance scores than those speaking face to face in the same class about the same situations. This supports the notion that writing has a positive effect in the learning process as well (see 4.3 above). Two important aspects of learning for understanding is the ability of the learner to take what has been learned and be able to transfer it to new situations and to utilize the learned material for a long time (not just until the test). Georghiades (2000; 2004) found that metacognitive instruction (discussing/reflecting, keeping diaries, concept mapping, and analyzing models) embedded within the fifth grade science curriculum increased both of these aspects significantly against those who received the same content material but without the 17 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu metacognitive activities. Interestingly, Georghiades (2000) found that students who received metacognitive instruction showed only slightly better learning than the control group immediately following instruction, but showed dramatically better retention of material when tested eight months later. 5.0 Pilot Study Implementation I implemented the unifying instructional model along with the instructor of record for a course during the spring semester of 2009. The instruction took place in the two sections of an introductory physical science class for preservice elementary teachers in an inclusive elementary education program at a large Research I university in the northeastern United States. There were 80 participants, 76 females and 4 males. Forty of the students were in one of the sections and taught by the instructor of record of the course. I taught the other 40 students in a separate section. Most students were first-year students and this was the second semester of a twosemester sequence in science and became participants by virtue of their being in the course. Our common planning helped ensure that we each taught both sections similarly. Enhancing these preservice teachers’ understanding of scientific concepts has far reaching implications. Science instruction has been shown to be more effective when teachers have a firm understanding of the content they are teaching. Higher quality elementary science instruction has also been shown to have a positive effect on students’ performance in science through high school. On the first day of the class, I introduced the students to several ideas. These included the use of models in science, the idea that they construct their own understandings of reality, and that their understanding is called their mental model. We discussed the use of such models to either describe a phenomenon or explain a phenomenon. I then had students record their mental 18 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu model of the nature of matter. We continued to incorporate activities which would demonstrate aspects of matter’s particulate nature for the next several weeks. Bereiter (2002) stated that “[i]t begins getting students on sufficiently intimate terms with the object to be understood that they can ask why questions with some meat” (p. 126, his italics). The object, or conceptual artifact, to be understood is the kinetic molecular theory. To do this, we started with an activity where students placed a couple of drops of vanilla extract into a balloon, inflated it, tied it off and then made observations of the balloon-vanilla-breath system. During the activity, the alcohol base of extract evaporated, permeated the latex, and students smelled the vanilla on the outside of the balloon. Students then developed a preliminary model (a diagram with written explanation) to explain their observations. Over the next few classes, and through similar activities and investigations, we introduced students to the properties and phases of matter. Students utilized and modified their models as a foundation for explaining their subsequent experiences. Students kept a science notebook where they collectively or independently recorded predictions, procedures, observations, and finally made evidence based claims. After completion of each activity, we directed students to revisit their mental model of matter to test it for congruency with what they had just observed. We also conducted small and whole group discussions about important aspects of the activities and demonstrations. We encouraged students to pose their questions and posit possible answers with rationales based on their working models. We had them record these reflections in their science notebook as well. There were very few truly experimental activities. We had preset many of the variables and students made predictions about reactions and justified those reactions based on their mental model. At the end of the series of investigations, we assessed students on a slide presentation that each small group made to the whole class. The “mediated artifact” consisted of a claim that the 19 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu group developed as a result of activities and the evidence to support their claim came from their observations during the activities. We collected students’ science notebooks, and using a rubric, checked them for completeness. We also assigned an essay asking students to make three claims about the particulate nature of matter from their mental model and to support each claim with evidence from the activities. Students also wrote a metacognitive piece describing the impact of the class activities and discussion on their mental model of matter. Finally, we gave students a final exam composed of open ended questions probing their understandings of various aspects of the nature of matter. The data we gathered came from science notebook entries, student essays describing their understanding of structure of matter as they compared and contrasted that new understanding with the understanding they started the class with. We also used responses to questions on the midterm exam and their class presentations - each consisting of a claim about the structure of matter with evidence. We looked for an evolution and utilization of students’ models, which were recorded and revised in their science notebooks, along the progression of activities. 5.1 Preliminary Findings I examined students’ science notebooks, essays and exams and allowed general themes in the data to emerge. Initial patterns in the data showed that students’ initial mental models were very simplistic and general. Students made statements such as, “Matter is everywhere,” Matter is everything that we see,” “Matter comes in three phases,” and “Matter exists as a solid, liquid, or gas.” There was minimal mention or indication in any of the responses that matter was made of tiny particles and that the behavior of these particles determined the behavior of the matter they made up. 20 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu The essay assignment and final exam drew these responses: I know that water, specifically, has a high capillary action because water particles stick to each other and other substances… …liquid takes on the shape of its container because the bonds between the molecules are not strong enough to hold it in one specific shape. I believe that when she pulls the syringe (of a confined gas) back, to 20ml the temperature should decrease because of the lower frequency of particle collisions. As can be seen from the examples, student ideas about matter show a definite increase toward sophistication. Instead of describing matter only in terms of it phases or by its ubiquitous nature, students have included references to the particle nature of matter. They also go on to describe that these particles interact with each other; they stick together or they collide against one another. Some explanations also utilized student-derived analogies as part of their mental models. Two examples that we noted were: A balloon served as a good example to describe the nature of a solid’s wholeness (or hole-ness): its material is composed by a pattern of overlapping threads, and though together they form a ‘solid’ balloon, among those woven rubber threads are tiny spaces which allowed the scent to travel through (my emphasis). Another way to think of matter as being mostly empty space is by comparing it to a chain link fence. A chain link fence is mostly empty space and human hands can be put 21 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu through it, however the ‘bonds’ of the fence are strong and it is hard to break, this is similar to the strength and tightness of matter. Since the analogies illustrated in the quotes above are not examples of analogies used in classroom discussion, this process of model construction must be a case of the students building this new knowledge into their personal prior knowledge framework, indicating more meaningful learning. 5.2 Student Metacognition Within their essays, we asked students to conclude by making and then supporting the claim that their mental model of matter had been impacted in some way due to instruction. The following are some quotes from different essays: I tried to recollect the images and the ideas that I remembered from high school science classes about properties and behaviors of matter, but all I could remember were simple ideas about the separate three phases. I had no complex beliefs about matter and didn’t make any statements about how the three stages of matter could relate to one another (student emphasis). My mental model of matter began very general and has expanded through experimentation and discussion with peers. Just by writing this paper I realized how much more information I know about the nature of matter now than I did on the first day. The process of writing this essay has further developed my model as well, because it forced me to analyze my claims and make them general, so that they are true not to just one specific experiment, but matter as a whole. 22 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu My mental model has greatly improved, coming from not even knowing what one was, to now formulating hypotheses from what I already possess in my mental model. (emphasis mine) Through this learning experience I discovered that I have a mental model that can be altered and added onto. By having students create a written record of what they thought about matter, prior to instruction, they had a reference point from which to gauge their progress through the course of instruction. Not only did some students become aware that they had a mental model, but that they could impact that mental model by relating their experiences to it and looking for coherence between model and observation. Some even gained insight into the fact that their mental model can be used to make hypotheses and predictions about phenomena. 5.3 Other Observations Another observation that we made as the course of instruction progressed was student inability to tell the difference between claims and evidence. Often, claims made by students were a restatement of their observations but without the data presented. For instance, students explored Boyle’s law where an amount of gas was placed into a syringe and hooked up to a pressure sensor so students could record pressure changes within the syringe resulting from changing the volume of the gas by pushing in or pulling out the plunger of the syringe. At the conclusion of the activity, many students made the claim that as the volume of a confined gas decreased, the pressure increased. They supported this claim with evidence which said the exact same thing, only with their empirical data. They did not seem to be able to connect this activity with any others that demonstrated how matter takes up space, or that the pressure is caused by 23 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu the force exerted by colliding particles. In their essays, although few were able to make claims about matter which they supported across a spectrum of activities, most made claims of a very limited nature, dealing with results of only one activity. This inability to make claims with broader implications is most likely related to a second limitation of their claim generation; most of the claims they made were strictly descriptive in nature. They described the relationship between different variables, namely pressure and volume of a gas, temperature and phase, and the reactions created when certain substance are mixed together. There were very few claims that were explanatory in nature. These would be claims revolving around the particulate nature of matter. In both classes, we discussed the idea of particles making up matter and showed various modes representing this concept. When expressed, models which utilized these few examples were isolated to the context in which they were introduced. There did not seem to be much carryover from one activity to the next. Another observation we made was the gradual emergence of certain misconceptions during the evolution of their mental models. One such misconception was their anthropomorphism of matter. After studying the behaviors of matter under different conditions, student descriptions of that behavior contained allusions to human behavior. Examples of this are demonstrated in the following quotes: “The vanilla traveled through the pores in the balloon to outside it, trying to equalize the concentration,” “The gas has the ability to travel through a solid,” and “Matter is capable of changing its form due to its surrounding environment.” Here, students have tried to give inanimate material decidedly human qualities as desire and capability to perform an action. Another interesting misconception developed as a direct result of instruction. We presented students with the idea of a chemical reaction being one where two or more substances, 24 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu when brought together, can react and create new substances. They explored a few examples of this and surmised that indications of such a chemical change can be a color change, the production of a gas, and a temperature change. A final demonstration involving phase changes utilized dry ice placed in water which was red from the added phenol red indicator. Of course the students observed violent bubbling from the sublimation of the dry ice. Carbon dioxide dissolving into the water turned the indicator from red to yellow, as the pH turned from neutral to acidic. Condensation began to form on the outside of the jar of water as energy was used to turn the dry ice into gaseous carbon dioxide. A significant number of students, when asked to describe the phenomenon on a test later, recorded the event as a chemical reaction, because it showed the indications of a chemical reaction, despite the fact that they also knew that there was no chemical change in the transformation from solid carbon dioxide to gas. 6.0 Implications for Teaching the Nature of Science Recent science education reform documents emphasize the importance of a scientifically literate population (AAAS, 1993; NRC, 1996). An aspect of science literacy is having a sound understanding of the nature of science (NOS) (Rutherford & Ahlgren, 1990; Nuhfer & Mosbrucker, 2007). Though NOS does not have a hard and fast definition, it encompasses ideas including how science as a process works, the epistemology of science, how scientists behave as a social group, and how science both affects and is affected by society. (For a discussion of these ideas, see Clough, 1997, McComas, Clough, and Almazroa, 1998, and Rutherford and Ahlgren ,1990). To enhance NOS instruction for students, these understandings need to become a facet of science teacher education. Lederman (1999) stated that teachers who know the importance of teaching NOS may be less likely to ignore this content when making their 25 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu instructional decisions. Abd-El-Khalick and Lederman (2000) draw an analogy between the importance of NOS cognitive outcomes and the outcomes aimed at general content understanding, and Ackerson and Abd-El-Khalick (2003) continue the analogy by emphasizing the importance of treating explicit NOS instruction within a teacher education program in relation to other content instructional strategies. However, Abd-El-Khalick (2001) found that NOS instruction was more effective with students in a science content course, as opposed to a science methods course. Although literature shows that students will not pick up NOS understandings from implicit, contextualized activities (facets of NOS “built in” to the content) alone (Khishfe, & Abd-El-Khalick, 2002; Lederman, 1999), what has shown effectiveness is intermittently utilizing decontextualized NOS activities – namely, black box activities or any other example of NOS understandings that are not connected to course content (Clough, 2003), and instruction which offers explicit (Khishfe, & Abd-El-Khalick, 2002) and prolonged (Clough, 1997) contextualized NOS activities. In his book comparing model construction in scientists to model construction in students, Clement (2008) makes the following claim: [M]any of the powerful reasoning and learning processes used by experts to achieve scientific understandings are also helpful in helping students learn scientific understandings. This parallel occurs in each of the three major categories of applying intuitively grounded knowledge, analogical reasoning, and model construction… (author’s emphasis) (p. 2) With the emphasis of the instructional cycle on model evolution, inquiry, development and justification of claims from observational data, the learning environment very much parallels 26 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu the learning taking place in the scientific community. It was with this in mind that we distributed a copy of the model of the instructional cycle to all students at the beginning of the semester, and again around the mid-term. In both instances, we reviewed the various steps along the cycle, the activities and goals at each stage, and discussed some of the parallels between how their scientific knowledge will be created and how scientific knowledge is created by scientists. Our rationale was that by having students become aware of and reflecting on how they are learning and that their learning parallels how scientists learn, they would have the necessary explicit and prolonged exposure to certain NOS concepts as called for by Khishfe, and Abd-El-Khalick (2002) and Clough (1997) to develop their NOS understandings. Additionally, the purposeful use of models within instruction can aid in meeting this end. Snir, Smith, and Raz (2003) argue “that a central element of science teaching should be about metaconcepts as well as relevant concepts. That is, students should understand what a model is and how it is used in science. We believe that if this is done constantly as each new concept is introduced in science, students…will be able to understand science as a way of thinking which they can apply to their everyday world” (p. 802). It was our hope to foster this outcome by utilizing and explaining the models during instruction and by repeatedly drawing students’ attention to their own model development and expression. 6.1 Explicit, Decontextualized Activities We employed a few decontectualized NOS activities and discussions to highlight several aspects of NOS. The first was an activity involving a cube with an arrangement of names and numbers on five sides. The sixth side was the bottom of the cube, and hidden from view. During the activity, students worked in groups and looked at the five exposed sides of a cube to 27 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu delineate any patterns. The object of the activity was for students to predict what was on the bottom of the cube. They were never allowed to see what was on the bottom, but only make a prediction and defend it to the rest of the class. We also discussed, through the use of optical illusions, how their senses, brain, and past experiences are sources of bias in making both observations and inferences. (For a more detailed description of this, see Remis and Dolphin, 2008). 6.2 Explicit, Contextualized Activities As part of the instruction, students received a list of five questions from the VNOS-C (Abd-El-Khalick, 1998; Lederman, Schwartz, Abd-El-Khalick, & Bell, 2001) all dealing with various NOS aspects (Figure 8). Questions focused on NOS understandings having to do with science as a discipline of inquiry, the acquisition of scientific knowledge, the tentative yet durable nature of scientific knowledge, and the difference between scientific laws and scientific theories. The purpose of the questions was to raise students’ awareness of their own mental model of science. We were explicit about this purpose with them. We asked them to answer the questions and to return to them weekly to confirm or modify their understandings based on their activities in class. Their final assignment was to address three of the five questions within an essay with claims about the nature of science supported by evidence from class work and discussions (Appendix A). Instruction concerning different types of models took place prior to the NOS questions. We discussed the importance of their mental models and how learning happens when their mental models change and grow in sophistication. We also explored the different purposes of models - one being to describe phenomena and the other being to explain phenomena. We drew 28 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu the connection between their descriptive models, such as describing the change in pressure of an enclosed gas when the volume if its container is changed, and scientific laws (i.e., Boyle’s Law). We also highlighted the connection between their explanation of why the gas pressure changes with volume and scientific theories (i.e., Kinetic Molecular Theory). Lastly, we emphasized that their mental models were the starting point of experimentation. Their experimental predictions would be based on this model. If the prediction withstood testing, then it would remain as is; if it failed, their model would have to be changed. Again, we sought student awareness of the parallels between their learning and how scientific knowledge is created. We distributed the NOS questions to the students at the mid-term of the semester, after the nature of matter unit, as we started instruction concerning force and motion. Most instruction was done through open inquiry, following the structure of the instructional model. Students explored concepts such as the physics of pendulums, floating and sinking, rubber band airplane flight and catapults. Students developed testable questions with predictions and rationalized them based on their prior understanding. In groups of three or four students, they designed and executed several experiments to test their predictions, all the while recording everything in their own science notebooks. After several class periods of experimentation, students developed claims that were supported by their accumulated evidence. They then created a mediated artifact in the form of a PowerPoint slide demonstrating their claim and evidence. The students, in their groups, then presented their claims to the rest of the class for discussion. 6.3 Student Learning and Science Knowledge Creation As part of their final exam, students were asked to draw a comparison between the process of their learning with that of scientific knowledge creation. After analyzing answers to 29 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu this question, some themes began to emerge with regard to student understanding of NOS. Students’ answers contained various aspects of both the acquisition metaphor and participation metaphor of learning, but there was only subtle evidence of the knowledge creation metaphor. Some student responses appear below. 6.3.a acquisition metaphor [A]fter finding that only the length of the pendulum affects the period we had to alter our mental models, and after scientists conduct experiments the reflect on their results and can make minor tweaks to their theories or claims. I did not throw out my first model of the nature of matter. I mearly [sic] added on specific information about the phases of matter that I learned in class. These students saw their learning as being individual. For instance, based on the comment above, this student changed her mental model by adding on to it based on what she learned in class; a process of the individual. The comments also reflect that this is how they see the creation of science knowledge where “scientists…make minor tweaks to their theories or claims” based on experimentation and reflection. 6.3.b participation metaphor In the scientific process, scientists often collaborate with one another to discuss their findings and/or debate them. As illustrated above, in class we used large group (class) discussions to clarify and expand our ideas. 30 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu In class, after dividing and experimenting, we regroup to discuss results. The collaboration allows for a group development of understanding of what’s been done, and individuals can contribute thoughts from their own mental model. These students recorded their awareness that the learning they came as a result of the group processes taking place in the class, and were able to relate that to the way scientists create new scientific knowledge as well. They saw the importance of discussions to “clarify and expand ideas” on the way to a “group development of understanding.” 6.3.c knowledge creation metaphor I had certain ideas in my head. I tested these ideas and explored my results, then discussed with my classmates. These discussions triggered new questions to explore and thus my learning continued (emphasis mine). This student did not see getting an answer or making a claim as an endpoint. She saw it as a springboard to new questions and continual learning. Although students in the small groups did work individually and cooperatively to create entries in their science notebooks and cooperatively on their class presentation of claims and evidence, they did not seem to internalize that the knowledge they created as part of their notebook or presentation was part of the learning process. It seems that their idea of making a presentation is showing what they know and not that they participated in a generative process where learning took place during the creation of the artifact. I was not explicit with students about how learning is mediated by the production of such artifacts. Klein (2004) argued that “without such explicit discussion, students may assume 31 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu that the purpose of a writing assignment is to improve their communication skills, or even to test their knowledge of a topic, rather than to learn” (p. 225). Students’ Views of the Nature of Science 6.4 As was stated earlier, we made explicit reference to the nature of science throughout the second half of the course through the use of a series of questions. The questions were to elicit their prior knowledge of the nature of science. We had them revisit the questions often to reassess their own understandings within the context of the activities they had done and the learning they had achieved. From the final essay they wrote recounting the evolution of their understandings of NOS, we observed positive changes in their understandings of what science was, how it worked, the difference between laws and theories, and the tentative, yet durable nature of scientific knowledge. Data in the form of student quotes from their essay support this claim. 6.4.a What is science? I depicted science as a static thing that was to be learned and strived for. However, now I view the nature of science as an ongoing, dynamic process that is systematic in its approach to gather observations for knowledge (emphasis in original). I used to think science was finding the answer…I now feel that science is a process of observation, finding patterns, making predictions, and continually adding to knowledge… I used to think that science was a way…to find absolute truths. I have now learned that science is in fact the ability to predict based on the advancement of our own mental models. 32 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu These quotes indicate an understanding of science as being a dynamic process; a process that ends not with ‘absolute truths” but continually adds to the knowledge base. Their descriptions of how science works, viz. “a process of observation, finding patterns, [and] making predictions reflects nicely the types of activities that the students participated in while performing the experiments during class. 6.4.b Ideas about Experiments I used to think that experiments were performed to prove something true, however now I know that the data we collect from experiments are use to support a claim. I used to think that an experiment was testing a hypothesis and controlling a test or investigation. I now understand that an experiment is a way of investigating a question and finding evidence to support a claim. Claims are models that we build on and our evidence is what we gain through observation. I also believed that an experiment was simply for finding evidence to support claims. I did not take into account the processes of observation and prediction. A common misconception about science is that the role of experiments is to “prove something true.” This was an idea held by most of the students. The quotes above indicate a softening of this type of scientism. These students see the goal of experiments as producing evidence that ultimately supports a claim, and that the claim is really a model built by humans. 6.4.c Law? Theory? What’s the difference? My own mental model holds examples of the differences between laws and theories. In my mental model, the explanatory claims I observed are theories. The presence of atoms 33 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu in my mental model, for example, is a theory. I cannot physically see atoms and molecules, but evidence about them has been previously gathered…My mental model does hold laws, however. Descriptive relationships, such as the relationship between string length and pendulum period in our pendulum experiment is a scientific law. This student eloquently draws the distinction between laws and theories, where laws are descriptions of physical relationships and theories are more explanatory and try to tell why such a relationship might exist. This is very different from the common misconception which sees scientific laws as being an absolute and unchanging truth; something that has been proved, and scientific theories which are seen as an educated guess waiting for enough evidence from testing, to eventually become a law. This student was also able to draw the parallel between her own descriptive and explanatory mental models and larger scale laws and theories. 6.4.d The Durability of Scientific Knowledge Although scientific knowledge is supported by an abundance of data from repeated trials, it is not considered the final answer. Scientific knowledge is flexible. Scientists continually test and challenge previous assumptions and findings. …the theory of evolution does not change, but fossil and othe[r] discoveries can enhance the theory and generate more knowledge. …scientific theories evolve as new technologies are made available. These quotes are examples that demonstrate student understanding of the durable, yet tentative nature of scientific knowledge. This is in mark contrast to the commonly held precept that scientific knowledge is ultimate and unchanging. Students show awareness that when new 34 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu information comes along that does not support the current scientific explanations, the explanations must be modified. Having had to do this with their own understanding through the course may have helped them to draw that conclusion. They also indicated an understanding that developments in technology could help to enhance abilities to observe, allowing for the new data to be collected. So as not to paint a completely rosy picture of results, similar to Abd-El-Khalick (2001) that the majority of students did not move just slightly from the idea that science was the uncovering of formal truths by completely objective scientists, to a more moderate understanding of the human aspects of scientists and the tentative yet durable nature of scientific knowledge. Instead they left the realm of scientism for the idea that “scientific knowledge as ‘someone’s opinion about what’s going on’” (Abd-El-Khalick, 2001, 229). We were able, however, by drawing attention to their won learning as a parallel to the creation of knowledge by scientists, to get them to see and articulate a more sophisticated view of the scientific process. 7.0 Discussion and Conclusions This pilot study has shown that the educational value of the proposed instructional model holds promise from both the standpoint of students learning in the content domain as well as in the domain of NOS understandings. There was an obvious increase in sophistication in students’ mental models regarding the particulate nature of matter. They were also able to recognize their simplistic understandings of science as being “static” and a “search for truth” and come to understand it as a process; a human endeavor. In some cases, the implications of science being “human,” namely its tentative nature, also came through in their writings and discussions. Students commented on many of the strategies incorporated within the instruction, namely small 35 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu group and whole class discussions, experimental activities where they were responsible for the design, classmates’ presentations, and reflective assignments, as being both instrumental in their learning and making the class challenging, yet enjoyable. The decision to implement the instructional cycle was very last minute, only because it was being developed just weeks before the beginning of the spring semester. This created a situation where some pedagogical decisions had to be made in real time – not the most efficient way to run a class. Though many of the teaching strategies presented here were already a part of the instructional repertoire, some shifting in activities and timing still had to take place. Hindsight, however, is usually 20/20 and there are some things that could be changed that could make the outcomes even more powerful. In general, students were not able to connect the fundamental principles demonstrated in the activities to one another. Information from each activity seemed to be compartmentalized and treated as discrete bits of knowledge. More attention should be paid by the instructor to the order of activities and the potential of scaffolding information better, creating a story line so to speak, where previous information is used to create new information. Student attention should be drawn to this explicitly as well. This could also help remediate students’ general lack of ability to make general claims which are supported by evidence. It seems that because they were drawing from only a discrete set of data to make their claims, their claims were much more descriptive than explanatory and much more limited in scope. It is very important to place a greater emphasis on explicitly informing students that the artifacts they are creating in their science notebooks and presenting to the class are not just learning activities in their own right, but that these conceptual artifacts can and should be used as tools for facilitating further understanding. This could be done by having the students reflect on 36 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu their creativity and gain awareness of when they’ve utilized newly gained knowledge to further their understanding. Also, I feel that referring them to the actual model of instruction more often could help them be more aware of the activities they are participating in and the rationale behind that participation so they might see how they are learning and take more control of that process. This could help them to strengthen the analogy between their knowledge gains and those gains made within the scientific community. They may also gain some insight into pedagogy so they can practice these types of strategies with their future classes. I observed many misconceptions from the students. A portion of which I feel were latent misconceptions, anthropomorphism for example, and were not expressed in their earlier mental models because their models were not sophisticated enough to support them. Other misconceptions seemed to be brought about from the actual instruction. This was observed with students considering the sublimation of dry ice as being a chemical change because they observed some of the some of the characteristic indications of a chemical change, regardless of the fact that they knew there was no change in the chemical nature of the substances involved. This may be a result of the almost purely descriptive nature of students’ models. Since their models were based mainly on observations, they lost the importance of a chemical reaction being one where substances change into new substances and the temperature, color change and gas production are merely indicators of such a change not the definition of the change. Von Glasersfeld (1996) argued that “The sensory objects, no matter how ingeniously they might be designed...no matter how trivial and obvious they might seem to the teacher, are never obvious to the novice" (p. 311). Only mindful implementation of activities and constant monitoring of student participation in their knowledge creation can help to minimize the occurrence of misconceptions and facilitate meaningful learning. 37 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu References cited Abd-El-Khalick, F. (1998). The influence of history of science courses on students’ conceptions of nature of science. Unpublished doctoral dissertation. Oregon State University, Corvallis. Abd-El-Khalick, F. (2001). Inbedding nature of science instruction in preservice elementary science courses: Abandoning scientism, but…Journal of Science Teacher Education 12(3), 215-233. Abd-El-Khalick, F., & Lederman, N. (2000). Improving science teachers’ conceptions of the nature of science: A critical review of the literature. International Journal of Science Education, 22, 665-701. Ackerson, V., & Abd-El-Khalick, F. (2003). Teaching elements of nature of science: A yearlong case study of a fourth-grade teacher. Journal of Research in Science Teaching, 40(10), 1025-1049. American Association for the Advancement of Science (AAAS) (2001). Atlas of science literacy: Project 2061. Washington D.C.: AAAS. & NSTA. American Association for the Advancement of Science (AAAS) (1993). Benchmarks for scientific literacy: Project 2061. Washington D.C.: AAAS. Anderson, R., Reder, L., & Simon, H. (1997). Situative versus cognitive perspectives: Form versus substance. Educational Researcher, 26(1), 18-21. Anderson, R., Reder, L., & Simon, H. (1996). Situated learning and education. Educational Researcher, 25(4), 18-21. Appleton, K. (1997). Analysis and description of students’ learning during science classes using a constructivist-based model. Journal of research in Science teaching, 34(3), 303-318. 38 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu Ardak, D., & Akaygun, S. (2005). Using static and dynamic visuals to represent chemical change at molecular level. International Journal of Science Education, 27(11), 12691298. Bereiter, C. (2002). Education and mind in the knowledge age. Hillsdale, NJ: Erlbaum. Boulter, C., & Buckley, B. (2000). Constructing a typology of models. In Gilbert, J. & Boulter, C., (Eds.), Developing Models in Science Education (41-57). Netherlands: Kluwer. Bybee, R., Taylor, J., Gardner, A., Van Scotter, P., Carlson Powel, J., Westbrook, A., et al. (2006). The BSCS 5E instructional model: Origins, effectiveness, and applications. Colorado Springs: BSCS. Retrieved from http://www.bscs.org/pdf/bscs5eexecsummary.pdf on 10.04.09. Clement, J. (2008). Creative model construction in scientists and students. Dordrecht: Springer. Clough, M. (2003). The nature of science: Understanding how the “game” of science is played. In Weld, J. (Ed.), The game of science education, (pp. 198-227). Allyn & Bacon. Clough, M. (1997). Strategies and activities for initiating and maintaining pressure on student’ naïve views concerning the nature of science. Interchange, 28(2), 191-204. Comacho, F., Gallegos-Cázares, L., Garritz, A., & García-Franco, A. (2007). Incommensurability and multiple models: Representations of the structure of matter in undergraduate chemistry students. Science & Education, 16(7-8), 775-800. Driver, R., Asoko, H., Leach, J., Mortimer, E., & Scott, P. (1994). Constructing scientific knowledge in the classroom. Educational Researcher, 23(7), 5-12. Else, M.J., Clement, J., & Rea-Remirez, M. (2008). Using analogies in science teaching and curriculum design: Some guidelines. In Clement, J., & Rea-Remirez, M., (Eds.) Model Based Learning and Instruction in Science (215-231). Dordrecht: Springer. 39 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu Engeström, Y. (1999). Innovative learning in work teams: Analyzing cycles of knowledge creation in practice. In Engeström, Y., Miettinen, R., & Punamäki, R. (Eds.), Perspectives on activity theory.(pp. 37-404). Cambridge, UK:Cambridge University Press. Flodin, V. (2009). The necessity of making visible concepts with multiple meanings in science education: The use of the gene concept in a biology textbook. Science & Education, 18(1), 73-94. Flores-Camacho, F., Gallegos-Cázares, L., Garritz, A., & García-Franco, A. (2007). Incommensurability and multiple models: Representations of the structure of matter in undergraduate chemistry students. Science & Education, 16, 775-800. Gagné, R., Briggs, L. & Wager, W. (1992). Principles of Instructional Design (4th Ed.). Fort Worth, TX: HBJ College Publishers. Georghiades, P. (2000). Beyond conceptual change learning in science: Focusing on transfer, durability and metacognition. Educational Research, 42(2), 119-139. Georghiades, P. (2004). Making pupils’ conceptions of electricity more durable by means of situated cognition. International Journal of Science Education, 26(1), 85-99. Gilbert, J., Boulter, C., & Elmer, R. (2000). Positioning models in science education and in design and technology education. In Gilbert, J. & Boulter, C., (Eds.), Developing Models in Science Education (3-17). Netherlands: Kluwer. Gobert, J. (2005). The effects of different learning tasks on model-building in plate tectonics: Diagramming versus explaining. Journal of Geoscience Education, 53(4), 444-455. Gobert, J. (2000). A typology of causal models for plate tectonics: Inferential power and barriers to understanding. International Journal of Science Education, 22(9), 937-977. 40 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu Gobert, J., & Clement, J. (1999). Effects of student generated diagrams versus student generated summaries on conceptual understanding of causal and dynamic knowledge of plate tectonics. Journal of Research in Science Teaching, 36(1), 39-53. Greeno, J.(1997). On claims that answer the wrong question. Educational Researcher 26(1), 517. Grosslight, L., Unger, C., Jay, E., & Smith, C. (1991). Understanding models and their use in science: Conceptions of middle and high school students and experts. Journal of Research in Science Teaching, 28(9), 199-822. Halloun, I. (2007). Mediated modeling in science education. Science & Education, 16, 653-697. Halloun, I. (2004). Modeling theory in science education. Netherlands: Kluwer. Keys, C. (2000). Investigating the thinking processes of eighth writers during the composition of a scientific laboratory report. Journal of Research in Science Teaching, 37(7), 676-690. Keys, C., Hand, B., Prain, V., & Collins, S. (1999). Using the science writing heuristic as a tool for learning from laboratory investigations in secondary science. Journal of Research in Science Teaching, 36(10), 1065-1084. Khan, S. (2008). Co-construction and model evolution in chemistry. In Clement, J., & ReaRemirez, M. (Eds.) Model Based Learning and Instruction in Science (59-78). Dordrecht: Springer. Khishfe, R., & Abd-El-Khalick, F. (2002). Influence of explicit and reflective versus implicit inquiry-oriented instruction on sixth grader’s views of nature of Science. Journal of Research in Science Teaching, 39(7), 551-578. Klein, P. (2004). Constructing scientific explanations through writing. Instructional Science, 32, 191-231. 41 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu Lederman, N. (1999). Teachers’ understanding of the nature of science and classroom practice: Factors that facilitate or impede the relationship. Journal of Research in Science Teaching, 36(8), 916-929. Lederman, N. G., Schwartz, R. S., Abd-El-Khalick, F., & Bell, R. L. (2001). Pre-service teachers' understanding and teaching of the nature of science: An intervention study. Canadian Journal of Science, Mathematics, and Technology Education, 1, 135-160. McComas, W., Clough, M., &Almazroa, A (1998). The role and character of the nature of science in science education. In McComas, W. (Ed.), The nature of science in science education. Netherlands: Kluwer. National Research Council (NRC) (1996). National science education standards (NSES). National Academies Press. http://books.napedu/readingroom/books/nses/html/ retrieved 11.04.09. Nonaaka, I., & Takeuchi, H. (1995). The knowledge creating company: How Japanese companies create the dynamics of innovation. New York: Oxford University Press. Novak, J. & Cañas, A. (2000). The theory underlying concept maps and how to construct them, Technical Report IHMC CmapTools 2006-01 Rev 01-2008, Florida Institute for Human and Machine Cognition, 2008", available at: http://cmap.ihmc.us/Publications/ResearchPapers/TheoryUnderlyingConceptMaps.pdf. Retrieved 06.04.09. Nuhfer, E., and Mosbrucker P, 2007, Developing Science Literacy using Interactive Engagements for Conceptual Understanding of Change through Time, Journal of Geoscience Education, vol. 55, no. 1, pp. 36-50. 42 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu Núñez -Oviedo, M., & Clement, J. (2008). A competition strategy and other modes for developing mental models in large group discussion. In Clement, J., & Rea-Remirez, M. (Eds.) Model Based Learning and Instruction in Science (215-231). Dordrecht: Springer. Núñez-Oviedo, M., Clement, J., & Rea-Remirez, M. (2008). Developing complex mental models in biology through model evolution. In Clement, J., & Rea-Remirez, M. (Eds.) Model Based Learning and Instruction in Science (215-231). Dordrecht: Springer. Paavola, S. & Hakkarainen, K. (2005). The knowledge creation metaphor – An emergent epistemological approach to learning. Science & Education, 14, 535-557. Paavola, S., Lipponen, L., & Hakkarainen, K. (2004). Models of innovative knowledge communities and three metaphors of learning. Review of Educational Research, 74(4), 557-576. Piaxão, I., Caldo, S., Ferreira, S., Alves, V., & Morais, A. (2004). Continental drift: A discussion strategy for secondary school. Science & Education, 13, 201-221. Pintrich, P. R., Marx, R. W., & Boyle, R. A. (1993). Beyond cold conceptual change: The role of motivational beliefs and classroom contextual factors in the process of conceptual change. Review of Educational Research, 63, 167-199. Posner, G., Strike, K., Hewson, P., & Gertzog, W. (1982). Accommodation of a scientific conception: Toward a theory of conceptual change. Science Education, 66(2), 211-227. Rea-Remirez, M. (2008). Determining target models and effective learning pathways for developing understanding of biological topics. In Clement, J., & Rea-Remirez, M. (Eds.) Model Based Learning and Instruction in Science (45-58). Dordrecht: Springer. 43 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu Rea-Remirez, M., & Núñez-Oviedo, M. (2008). Role of discrepant questioning leading to model element modification. In Clement, J., & Rea-Remirez, M. (Eds.) Model Based Learning and Instruction in Science (195-214). Dordrecht: Springer. Remis, R., and Dolphin, G. (2008). Two birds…one stone: A conversation of the importance of the nature of science (NOS) and how it parallels literacy in the science classroom. The Science Teachers Bulletin, 71(2), 4-10. Rutherford, F., & Ahlgren, A. (1990), Science for all americans. New York, Oxford University Press. Sfard, A (1997). On two metaphors of learning and the dangers of choosing just one. Educational Researcher, 27(2), 4-13. Smith, C., Maclin, D., Grosslight, L., & Davis, H. (1997). Teaching for understanding: A study of students’ preinstruction theories of matter and a comparison of the effectiveness of two approaches to teaching about matter and density. Cognition and Instruction 15(3), 317393. Snir, J., Smith, C., &Raz, G. (2003). Linking phenomena with competing underlying models: A software tool for introducing students to the particulate model of matter. Science Education, 87, 794-830. Syh-Jong, J (2007). A study of students’ construction of science knowledge: Talk and writing in a collaborative group. Educational Research, 49(1), 65-81. Strike, K. A., & Posner, G. J. (1992). A revisionist theory of conceptual change. In R. Duschl & R. Hamilton (Eds.), Philosophy of Science, Cognitive Psychology, and Educational Theory and Practice (pp. 147-176). Albany, NY:SUNY. 44 ASTE Conference – January 2010 Glenn Dolphin – Syracuse University grdolphi@syr.edu Taber, K. (2003). Mediating mental models of metals: Acknowledging the priority of the learner’s prior learning. Science Education, 87, 732-758. von Glasersfeld (1996). Aspects of radical constructivism and its educational recommendations. In Steffe, L. P., Nesher, P., Cobb, P., Goldin, G. A., & Greer, B. (Eds.), Theories of mathematical learning. Hillsdale, NJ: Lawrence Erlbaum Associates. Zion, M., Michalsky, T., & Mevarech, Z. (2005). The effects of metacognitive instruction embedded within an asynchronous learning network on scientific inquiry skills. International Journal of Science Education, 27(8), 957-983. 45