Water Systems on Earth
advertisement

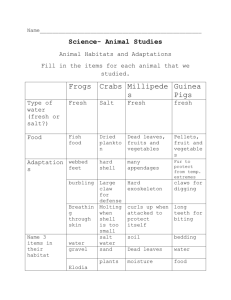
Water Systems on Earth Salt and Fresh water systems; differences and similarities What questions do you have about the pictures and captions below? Cost of 750 ml bottle of water = $1.45 Cost of 1 litre of gasoline = $1.30 Water is the lifeblood of Earth 70% of the Earth’s surface is WATER!! Of this 70%.... •97.2% is salt water •2.8% is fresh water This 2.8% is made up of approximately…. •2.15% ice (incl. glaciers) •0.61% ground water •0.01% lakes and rivers (the available H20) •0.001% atmosphere (in clouds, gas) The Water Cycle – where fresh water comes from… Remember – water exists in three states: Liquid (rain, standing water) Solid (ice) Gas (water vapour) Life on Earth depends on the Water Cycle Water cycle worksheet activity in package Differences between salt water and fresh water 1) Salinity 2) Density 3) Freezing point Salinity Salt water is named for the obvious reason that it has dissolved salt in it. However, fresh water also contains very small amounts of salt. We use the term SALINITY to discuss how much dissolved salt there are in a body of water. The water in the world’s oceans has an approximate salinity of 35 ppt (parts per thousand) That’s the same as dissolving 35g of salt in 1L of water. Freshwater has a salinity of less than 0.5 ppt. If you wanted to show this salinity how many grams of salt would you put into 1 L of water? Not all of the salt water that makes up the ocean has the same salinity. Where does salt water come from? Water seeps through the land and picks up dissolved solids over millions of years The dissolved solids in highest abundance are Na+ and Cl- (ions that easily bond together to make SALT!) The motion of the water easily mixes them together Volcanoes (undersea and on land) have high levels of dissolved solids and molten rock that seep into the ocean. As you can see in the diagram to the right that most of a salt crystal is made up of Cl- and Na+ but there are also other minerals in small amounts Density What is density? The measure of the amount of mass a given volume holds Ex. A sponge has a lower density that a rock of the same size. Ex. A bowling ball has a higher density than a volleyball of the same size Density - continued Why is salt water MORE DENSE than fresh water? Because the salt dissolved in it gives it more mass compared to it’s volume! Salt water has a density of approximately 1027 kg/m3 (which means that if you had 1m3 of salt water it would weigh 1027 kg) and fresh water has a density of approx. 1000 kg/m3. That’s why it’s easier to float in salt water….. there is more dissolved in the water (more density) to keep your body afloat! The Dead Sea A tourist takes advantage of the buoyancy in the Dead sea, which has an unusually high salinity of 6 times that of the Ocean because it does not have any outflow, only evaporation. (which as we learned earlier increases salinity!) Freezing Point Freezing point is directly related to the salinity…. Because the salt increases the density it lowers the freezing point. The more dense a material is the lower the freezing point. While fresh water has the obvious freezing point of 0 degrees Celsius, the freezing point of salt water is -1.9 degrees Celsius.