1-Fundamentals 2007W
advertisement

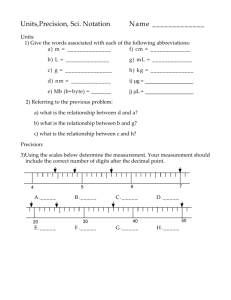
Scientific & Chemical Fundamentals Dr. Ron Rusay Fall 2007 © Copyright 2003-2007 R.J. Rusay Scientific & Chemical Fundamentals 1 Chemistry & the Scientific Method Matter : Classification & Properties Mathematics / Arithmetic: Exponents, Significant Figures Measurement & Units: (SI & metric) Conversions and Relationships: Dimensional Analysis: Density, Percent VOCABULARY: Key Terms, Bold Style Learning © Copyright 1998-2007 R.J. Rusay Textbook Reading Chemical Foundations 1.1 Chemistry: An Overview 1.2 The Scientific Method 1.3 Units of Measurement 1.4 Uncertainty in Measurement 1.5 Significant Figures and Calculations 1.6 Dimensional Analysis 1.7 Temperature 1.8 Density 1.9 Classification of Matter Science & The Scientific Method Law vs. Theory QUESTION The difference between a scientific law and a scientific theory can, at times, be confusing. For example, we will refer to the “Atomic theory” or perhaps the “Law of Gravity.” Should the Law of Gravity be changed to the Theory of Gravity? 1. Yes, no one can see gravity, it is better described as a theory. 2. No, scientific laws are based on summaries of many observations and gravity observations are well known and predictable. 3. Yes, gravity is better described as a theory because gravity explains why masses attract each other and theories are about explaining observations. 4. No, keep it as a law, laws offer explanations and gravity explains why masses attract each other and laws are about explaining observations. Some Possible Steps in the Scientific Method 1. • • 2. Observations qualitative quantitative Formulating hypotheses • possible explanation(s) for the observation 3. • • Performing experiments 4. Developing a theory Testing & Refining 5. gathering new information testing whether the hypotheses are valid Chemistry: The Study of Matter In all of its forms & all of its behaviors Sub-categories (not so distinct any longer) • • • • • • • • Organic: carbon Inorganic: non-carbon Organometallic: organic + inorganic Analytical: what?, how much?, how pure? Biological / Biochemistry: living organisms Physical: energy, changes, rates Nuclear: the nucleus Environmental: interdisciplinary, eg. Oceanography © Copyright 1998-2007 R.J. Rusay Chemistry & Matter (Chemicals) How many different chemicals do you think have been reported in the scientific literature? A) 100,000 B) 1,000,000 C) 10,000,000 D) 100,000,000 E) 1,000,000,000 Chemistry & Matter: Properties & States 1 • Physical vs. Chemical Properties • Solid (s), Liquid (l), Gas (g) • Homogeneous vs. Heterogeneous Mixtures • Organization of atoms/molecules: atoms/elements molecules/compounds • Extensive vs. Intensive Properties Varies with amount (extensive) or does not vary with amount (intensive) © Copyright 1998-2007 R.J. Rusay Observations of Physical & Chemical Properties QuickTime™ and a Cinepak decompressor are needed to see this picture. States of Matter QuickTime™ and a Cinepak decompressor are needed to see this picture. 01_15 Matter Organization of Matter Heterogeneous mixtures Physical methods Homogeneous mixtures (solutions) Physical methods Pure substances Classification of Matter Compounds Chemical methods Elements Atoms Nucleus Electrons Protons Neutrons Quarks Quarks Up,down, strange, charm, bottom, top leptons 01_15 Matter Organization of Matter Heterogeneous mixtures Physical methods Homogeneous mixtures (solutions) Physical methods Pure substances Classification of Matter Compounds Chemical methods Elements Atoms Nucleus Electrons Protons Neutrons Quarks Quarks up,down, strange, charm, bottom, top leptons muons, tau, neutrinos Using Physical & Chemical Properties: Distinguishing a Compound & a Mixture QuickTime™ and a Sorenson Video decompressor are needed to see this picture. The effects of a magnet on iron: filings in a mixture and atoms in a molecule. Types of Mixtures Mixtures have variable composition of two or more components. A homogeneous mixture is a solution (for example, vinegar: water + acetic acid, or steel & bronze: solid metals) A heterogeneous mixture is, to the naked eye, clearly not uniform (for example, a bottle of ranch dressing with two layers: water + oil, or two solids: iron and sulfur) Elements & Compounds Element: A substance that cannot be broken into simpler substances by chemical means, eg. Fe, Iron or S8 Sulfur Compound: A substance with a constant composition that can be broken down into elements only by chemical processes,eg. FeS, Iron (II) sulfide QUESTION Is a cup of coffee a homogeneous solution or a compound? Which of the following agrees with your reasoning? 1. The coffee in the cup is a homogeneous solution because it contains the same components throughout, but there are many compounds dissolved to make coffee. 2. The coffee in the cup is a compound because it has a set ratio of components that make it the same throughout. 3. The coffee in the cup is both a compound and a solution. It looks the same throughout like a true solution, yet it always has the same amount of each component. 4. The coffee in the cup is a heterogeneous solution not homogeneous because it contains distinct, different compounds dissolved to make coffee. Measurement & Units (SI units & common units in General Chemistry) 1 • Quantitative vs. Qualitative • MASS (Chem: gram; SI: kg) • LENGTH (Chem: cm & others; SI: m) • TEMPERATURE (Celsius & Kelvin; SI: K) • VOLUME (Chem: mL; SI: Liter) • CHEMICAL AMOUNT: Mole (mol) © Copyright 1998-2007 R.J. Rusay Units of Measure Units U.S. SI Chemistry Mass (weight) Pound (lb) Kilogram (kg) Volume Gallon (gal) u Liter (L) Temperature Fahrenheit (oF) Mile (mi), Feet(ft), Inches (in) Kelvin (K) “ Gram” (g, mg) “ Liter” (mL, L) K & Celsius (oC) Length Time Meter (m) “ Meter” (cm, mm, nm) Second (s) Second (s) Mole (mol) Mass and Volume Measurement Mass Determination (Weighing Devices: Balances) Volumes of regular shapes V=s3 h V=lxwxh 01_05 1m3 Volume 1dm3= 1 L 1cm3= 1 mL 1 cm 1 cm Liquid Measurement Tools Numbers & Measurement The Importance of Units Measurement - quantitative observation consisting of 2 parts • Part 1 - number • Part 2 - unit Examples: • 20 grams • 6.63 joules / second Scale: Size & Comparison Macroscopic vs. Microscopic IBM financed Video: http://www.wordwizz.com/imagendx.htm How would you compare your lifespan?.. to that of a dog? ….to the age of the earth?…How about the age of mankind to that of all life?.. ..the age of industrialized mankind to the age of mankind? Graphic Comparisons Powers of Ten: Scale Language describes scale (prefixes) Shorthand Prefixes How many zeroes does yotta yotta yotta have? Commonly used prefixes in Chemistry These should be known from memory. Commonly used prefixes in Chemistry The dark ones should be known from memory. QUESTION Conveniently, a U.S. nickel has a mass of approximately 5 grams. If you had one dollar’s worth of nickels what would be the mass of the nickels in milligrams? 1. 2. 3. 4. 100 milligrams 50 milligrams 1,000 milligrams 100,000 milligrams 1000 milligrams (mg) = 1 gram (g) Scientific Notation & Significant Digits Scientific Notation: A single digit followed by a decimal and a power of ten. Examples: 2,345 mL and 0.002340 g 2,345 mL = 2.345 x 10 3mL 0.002340 g = 2.340 x 10 -3 g Numbers 1 • Expressing a number correctly is determined by the method used in the measurement! • How many numbers should I include? Significant Digits (Figures) Consider: the exactness of the measured value • Short Hand expression translates the number: Scientific Notation © Copyright 1998-2007 R.J. Rusay What is the length of the rod? Different measurement tools give different numbers: Which ruler is better? ? cm 4.2 - 4.3cm ? cm 4.24 - 4.25cm What is the diameter of a circle? All measuring devices are not the same, and the values (numbers) that come from them indicate their limitations. Is there a better instrument to use other than a ruler? Buret 08 mL 0 10 22.2 mL 20 30 40 50 What does each line represent? 1 mL What can be estimated? O.1 mL Measurement Assignment http://chemconnections.llnl.gov/General/Chem120/volume1.htm Temperature Scales Relative to Water “Normal” Body Temperature QUESTION Dr. R. walks into class and claims, “It is very cold in here today. It feels like 242 K.” If that were the temperature, would you agree that you would feel cold? What would that be in Celsius degrees? 1. 2. 3. 4. I agree, that would be 31°C. I agree, that would be – 31°C. I do not agree, that would be 31°C. I agree, that would be –31.15°C. Temperature Precision & Accuracy (a) (b) (c) QUESTIONS: 1) Rank the images from best to worst precision. c>b>a 2) Rank the images from best to worst accuracy. c>a>b QUESTION Two Chem 120 students are each drinking a soft drink after class. The volumes of both containers are respectively listed as 375 milliliters. Philip remarks that the law requires bottlers to be very precise. Susan correctly responded: 1. If precision were the only requirement, bottlers could claim any volume as long as it was always very nearly the same volume. 2. Since precision is a requirement, bottlers have to get exactly 375 mL in every can. 3. Bottlers must have a precise average of all of the containers in a case of soft drinks equal to 375 mL. 4. If there were a difference of no more than +/- 1 mL between containers, the bottlers can sell their beverage. Precision & Accuracy Numerical Data a) b) a) 9.5 2 9.52 8.3 6 8.36 7.2 9 7.29 8.3 4 8.34 ___ ____ _____ ____ ____ Averag eAvera ge 8.378 Roun d Off Avera ge Roun d Off 7.9 5 7.95 8.0 0 8.00 8.0 5 8.05 7.9 5 7.95 ___ ____ _____ ____ ____ 7.988 8.38 7.99 9.52 8.36 7.29 8.34 __ ____ ____ 8.378 b) de vi atio n -1.14 0.02 1.09 0.04 0.573 8.38 +/- 0.57 c) 8.4 0 8.40 8.3 5 8.35 8.4 2 8.42 8.3 6 8.36 ___ ____ _____ ____ ____ 8.383 8.38 a) c) b) 8.40 8.35 8.42 8.36 __ ____ ____ 8.383 c) de vi atio n -0.02 0.03 -0.04 0.02 0.028 8.38 +/- 0.0 3 Abs olute va lue ( all of the - becom e +) de vi atio n 7.95 8.00 8.05 7.95 __ ____ ____ 7.988 7.99 0.0 4 -0.0 1 -0.0 6 0.0 4 0.038 +/- 0.04 QUESTION Rank the relative precision of the three sets of data: a), b) and c).The accepted value is 8.08. Average Average Average a) b) c) 8.38 8.38 7.99 average average average deviation deviation deviation a) b) c) +/- 0.57 +/- 0.03 +/- 0.04 A) Precision: a > c > b B) Precision: b > c > a C) Precision: a = b > c D) Precision: a > b > c QUESTION Rank the relative accuracy of the three sets of data: a), b) and c).The accepted value is 8.08. Average Average Average a) b) c) 8.38 8.38 7.99 average average average deviation deviation deviation a) b) c) +/- 0.57 +/- 0.03 +/- 0.04 A) Accuracy: a > c > b B) Accuracy: b > c > a C) Accuracy: c > a = b D) Accuracy: a = b > c Reporting Numbers Rules for Significant Digits (Figures) Nonzero integers always count as significant figures. 3456 g has how many sig figs? 4 sig figs. • Expressed in scientific notation? 3.456 x 10 3 g Reporting Numbers Rules for Significant (Digits) Figures Exact numbers (unit, conversion or scale factors) can have an infinite number of significant figures. 1 liter = 1,000. ml, exactly 1 inch = 2.54 cm, exactly Systematic Problem Solving Dimensional/Unit Analysis How many mL of milk are in a1/2 gallon carton? 0.50 gal 1 gal = 4 qt ? mL 1 qt = 946 mL 0.50 gal | 1 qt | 946 mL = ? mL | 4 gal | 1 qt Complete the following Units & Conversions Number 13,000,000,000 yrs. Scientific Notation 10 yrs 1.3 x 10 ________________ 546 ___________ mL 5.46 X 10 2 mL ______________ 0.845 ____________ kg __8.45 x 10 -1 kg___ Named unit __? gigayears 13 Gyrs 0.546 Liters 0.546 L _? grams__ 845 g Zeros Leading zeros do not count as significant figures. 0.0486 mL has how many sig figs? 3 sig figs. • Number expressed in scientific notation? 4.86 x 10 -2 mL Zeros Captive zeros always count as significant figures. 16.07 cm has how many sig figs? 4 sig figs. Number expressed in scientific 1.607 x 10 1 cm notation? Zeros Trailing zeros are significant only if the number contains a decimal point. 9.300 kg has how many sig figs? 4 sig figs. • Number expressed in scientific 9.300 kg notation? QUESTION Which one of the following does NOT represent a result with four significant digits? 1. 2. 3. 4. 0.07100 0.7100 0.7010 0.0710 Mathematics & Arithmetic 1 • Relative to method(s) of measurement • Short Hand expression: Scientific Notation • Numbers : How many to include? Quantitative vs. Qualitative • Addition/Subtraction...... • Multiplication/Division..... • What is “significant”?.....Rounding Off • http:dbhs.wvusd.k12.ca.us/SigFigsFable.html © Copyright 1998-2007 R.J. Rusay Computational Rules 1 • Addition/Subtraction: Answer expressed • to the least number of decimal places of the figures in the process Multiplication/Division: Answer expressed to the least number of significant figures © Copyright 1998-2007 R.J. Rusay Addition Four students were each asked to measure a piece of wire and provide a total length for the four pieces. Report the result correctly: 0.05 cm 12.01 cm 1.9 cm + 2.386 cm _______ 16.346 cm QUESTION If you were unloading a 23.50 kg box of books from your car and a “friend” added two more 482 gram chemistry books, how much in kg and using the rules for significant digits, would you be lifting? 1. 2. 3. 4. 23.98 kg 24.464 kg 24.46 kg 24.5 kg Mathematical Processes: Provide correct answers assuming each value (unit omitted) is written with the correct number of sig figs: 12.01 x 1.90 _______ ______________ = 9.56370 2.386 12.01 x 1.90 _______ ______________ + 0.05 = 9.61370 2.386 QUESTION The average mass of a certain brand of vitamin C tablets is 253 mg. What is the mass of three such tablets rounded to the proper number of significant digits? 1. 2. 3. 4. 0.760 grams 0.759 grams 0.7590 grams 0.253 grams Conversion Factor Method (Dimensional Analysis) 1 • Qualitative Descriptions vs. Quantitative • Use exact numbers / “scale factor” UNITS • A Bookkeeping Method: Example 5 ft___in 5 ___ --------> ? m • (1 ft = 12 in; 2.54 cm = 1 in; 100 cm = 1 m) 5 5 • ___ft x 12 in/ft + ___in = 65 ___in 1.651 65 • ___in x 2.54 cm/in x 1 m/100cm = ___m © Copyright 1998-2007 R.J. Rusay Density • Density = Mass / Volume [g/mL or g/cm ; g/L] • Least dense man-made solid substance: 3 1 Aerogel, D = 3.025 x 10-3 g/cm3 http://eetd.lbl.gov/ECS/aerogels/aerogels.htm http://stardust.jpl.nasa.gov/spacecraft/aerogel.html • D = 1.22 x 10 g/cm (1.22 g/L) • Densest known substance: a White Dwarf -3 air 3 http://antwrp.gsfc.nasa.gov/apod/ap961203.html 1.0 teaspoon = 3.0 T; D = ? g/cm3 (1 tsp = 4.93 mL; 1 mL = 1 cm3 ) © Copyright 1998-2007 R.J. Rusay QUESTION Which would provide more grams of NaCl, sample one with a mass of 2,350 mg, or sample two, a solid with a volume of 2.00 cm3? (The density of solid salt is 2.16 g/cm3.) Report your choice and report the grams of the more massive sample. 1. 2. 3. 4. Sample two; 1.08 grams Sample two; 4.32 grams Sample one; 2.35 grams Sample one; 2.350 grams Densities of Various Common Substances* at 20° C QUESTION The volume of a sample can be obtained from its density and mass. If the mass of a sample of acid from a battery were 5.00 grams and the density was 1.2 g/mL, what would you report in mL and with the proper number of significant digits, as the sample volume? 1. 2. 3. 4. 6.0 mL 6.00 mL 4.2 mL 4.17 mL Percent 1 • A comparison based on normalization to 100. • George Washington University: 64 unsealed addressed envelopes with $10 in each were dropped on campus in different classrooms. • In economics 18 of 32 were mailed, in business, history and psychology 10 of 32 were mailed. What is the percent for each of the 2 groups of students? © Copyright 1998-2007 R.J. Rusay Percent Continued 1 • The Professor conducting the study received 43.75% of the $640 in the mail. How much did he receive? • How many of you would mail the envelop presuming no one knows you found it? • One student mailed an empty envelop with the return address: Mr. IOU, 1013 Indebted Lane, Bankrupt City, MS (WSJ © Copyright 1998-2007 R.J. Rusay 1/18/95)