Mole Conversions Worksheet: Chemistry Practice Problems
advertisement
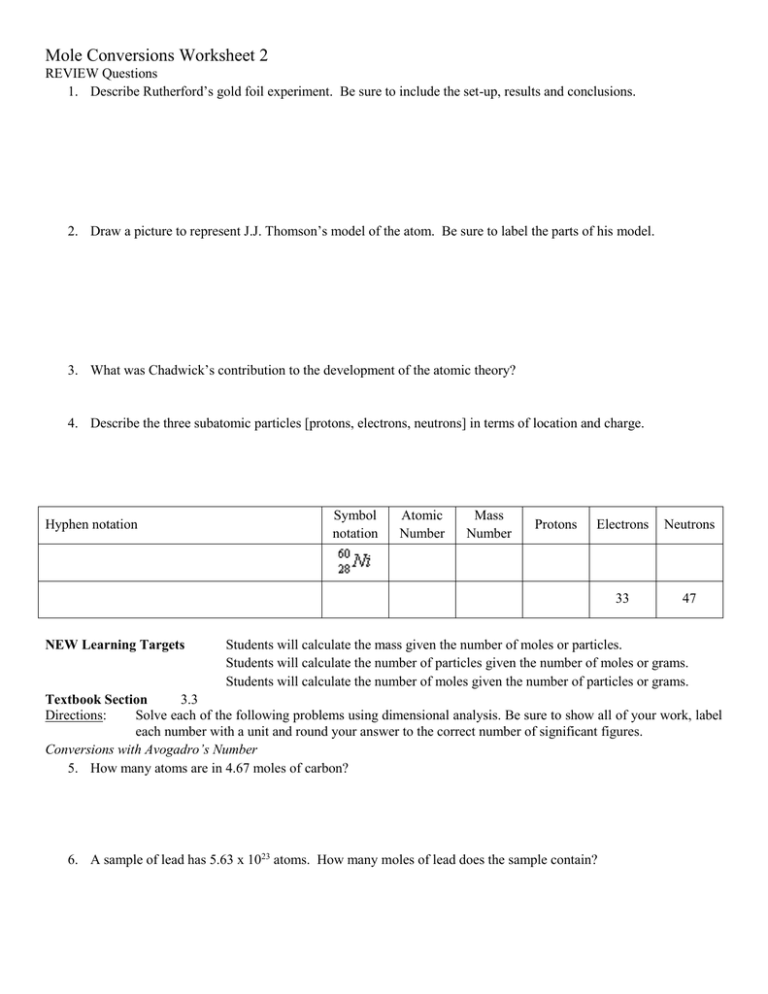
Mole Conversions Worksheet 2 REVIEW Questions 1. Describe Rutherford’s gold foil experiment. Be sure to include the set-up, results and conclusions. 2. Draw a picture to represent J.J. Thomson’s model of the atom. Be sure to label the parts of his model. 3. What was Chadwick’s contribution to the development of the atomic theory? 4. Describe the three subatomic particles [protons, electrons, neutrons] in terms of location and charge. Hyphen notation NEW Learning Targets Symbol notation Atomic Number Mass Number Protons Electrons Neutrons 33 47 Students will calculate the mass given the number of moles or particles. Students will calculate the number of particles given the number of moles or grams. Students will calculate the number of moles given the number of particles or grams. Textbook Section 3.3 Directions: Solve each of the following problems using dimensional analysis. Be sure to show all of your work, label each number with a unit and round your answer to the correct number of significant figures. Conversions with Avogadro’s Number 5. How many atoms are in 4.67 moles of carbon? 6. A sample of lead has 5.63 x 1023 atoms. How many moles of lead does the sample contain? 7. How many moles of platinum are in 4.9 x 1022 atoms of the element? 8. How many atoms are in 13 moles of the element aluminum? Conversions combining Avogadro’s Number with Molar Mass 9. How many grams are in 2.35 x 1025 atoms of sodium? 10. How many atoms of sulfur are present in a sample of the element with a mass of 2.3 grams? 11. How many atoms of arsenic are present in a 0.985 g sample of the element? 12. Determine the mass, in grams, of 1.9 x 1021 atoms of potassium. 13. What is the mass, in grams, of 9.75 x 1026 atoms of lithium? Mixed Conversions 14. Determine the mass of 12.4 mol of boron. 15. Determine the mass of 2.4 x 1024 atoms of carbon. 16. How many atoms are present in 0.756 g of beryllium? 17. Determine the number of atoms present in 1.75 moles of calcium. 18. Determine the number of moles present in a 1.75 g sample of barium. 19. CHALLENGE: How many atoms are present in 125 mL of magnesium, given that the density of magnesium is 1.74 g/mL? Answers 5. 2.81 x 1024 atoms carbon 6. 0.935 mol lead 7. 0.081 mol platinum 8. 7.8 x 1024 atoms aluminum 9. 897 g sodium 10. 4.3 x 1022 atoms sulfur 11. 7.92 x 1021 atoms arsenic 12. 0.12 g potassium 13. 14. 15. 16. 17. 18. 19. 11,200 g lithium 134 g boron 48 g carbon 5.05 x 1022 atoms beryllium 1.05 x 1024 atoms calcium 0.0127 mol barium 5.39 x 1024 atoms magnesium




