X-ray Spectrometry - Villanova University
advertisement

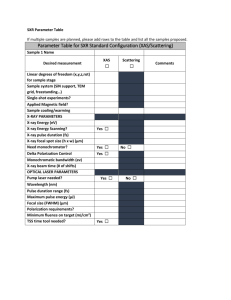
X-ray Spectrometry Lecture Date: February 4th, 2008 Notes See Chapters 12 and 21 (mostly Chapter 12) of Skoog This lecture covers both atomic and molecular applications of X-ray spectrometry X-ray diffraction is only briefly discussed here - it is covered in its own lecture along with its applications to crystallography and solid-state structural analysis Surface analysis and microscopy is also briefly discussed in advance of its own lecture Outline X-ray absorption/fluorescence processes – Auger electron emission – Photoelectron emission Excitation of X-rays – X-ray fluorescence, X-ray emission X-ray Detection and Spectrometer Design – Energy-dispersive (ED) spectrometers – Wavelength-dispersive (WD) spectrometers Methods and Applications Topics mentioned here but discussed in detail during the Surface Analysis and Microscopy Lecture: – Scanning electron microscopy – an X-ray emission “microprobe” – Auger electron spectrometry (electron energy) – X-ray photoelectron spectrometry (again, electron energy) The Electromagnetic Spectrum X-rays (Also gamma rays) X-rays What are X-rays? High energy photons. – Note: gamma rays are just high-energy X-rays Advantages of X-ray spectrometric methods: – The X-ray spectrum is not very sensitive to molecular effects or chemical state, or excitation conditions This is because core electrons are usually involved in X-ray transitions – physical and chemical state have only minute effects (I.e. gas vs solid, oxide vs. element). – Atomization is not necessary for elemental analysis – Precision and accuracy are good, spectra are simple – Surface-sensitive (penetration of 100 um at most) Disadvantages of X-ray methods: – Surface-sensitive, if you want bulk analysis (often not a problem) – Modest limits of detection, compared to other elemental methods (e.g. AA, ICP-OES, ICP-MS) X-ray Production X-ray are commonly produced by bombarding a target with electrons The target emits a spectrum with two components: – Characteristic radiation – Continuous radiation (also called white radiation, Bremsstrahlung (braking radiation) The Duane-Hunt limit explains the “cutoff” of the continuous radiation: eV0 hc min h max (where V0 is the electron accelerating voltage) X-ray Generation: Characteristic Radiation The characteristics lines in X-ray spectra result from electronic transitions between inner atomic orbitals The X-ray spectra for most heavy elements are much simpler than the UV/Vis spectra observed in ICP-OES, for example. (Only a few lines!!!) Big difference between X-ray and UVVis: The radiation is ionizing, and doesn’t just excite electrons to higher levels. Moseley’s law: Predicts the basic relationship of atom number and the frequency of the characteristic lines K Z where Z is the atomic number, and K and are constants that vary with the spectral series. X-ray Processes: when an X-ray strikes an atom… X-ray Generation: Characteristic Radiation X-ray transitions: (Here denoted using the Siegbahn notation) Remember the quantum numbers: n – principal quantum number l – angular momentum quantum number s – spin quantum number (1 and 2 have s = -1/2 and s = +1/2) j – “inner” quantum number, from coupling of l and s X-ray Generation: Characteristic Radiation X-ray transitions, for gold (Z=79), using both optical and X-ray (Siegbahn) notation. X-ray Generation: Nomenclature Example notations for Copper (K series) in different notations Transition Siegbahn IUPAC 2p3/2 1s K1 KL3 2p1/2 1s K2 KL2 3p3/2 1s K1 KM3 3p1/2 1s K3 KM2 R. Jenkins, et al., Pure Appl. Chem., 63, 736-746 (1991). X-ray Generation: Characteristic Radiation X-ray Generation: X-ray Tubes X-ray tubes: fire electrons at targets that are selected for their x-ray emission properties as well as their robustness, heat conductivity, etc… (Note – modern tubes are more efficient, no water cooling needed) X-ray Generation: The Future Goals – – – – Short pulsed sources (femtoseconds) Brilliant sources Coherent Small beam sizes One way of getting there… capillary optics (polycapillary lenses) – Achieve a higher spectral efficiency and small spot size for a given X-ray beam – Best as of 2004 – 19 keV focussed onto a 20-30 um spot I. Szaloki, J. Osan, and R. E. Van Grieken, “X-ray Spectrometry”, Anal. Chem., 76, 3445-3470 (2004). Design of X-ray Instrumentation Two major types: – Wavelength dispersive spectrometers Analogous to dispersive spectrometers encountered in IR and UVVis spectroscopy Radiation Source Wavelength Selector Sample Detector – Energy dispersive spectrometers No real analogy in dispersive spectrometry Detects portions of a spectrum directly through its energy Radiation Source Sample Detector Design of X-ray Instrumentation Most substances have refractive indices of unity (1) at Xray frequencies. – The reason – X-radiation is so high-frequency that there is no time for the electronic polarization needed to cause a refractive index…. Therefore, mirrors and lenses for X-rays cannot be made (in general), and other ways to control X-rays must be found X-rays can be diffracted by crystals…. – Compare this to the rulings and gratings used in optical spectroscopy – the wavelength of X-rays is so short, that only “molecular” diffraction gratings (crystals) can be used. Energy-Dispersive Analyzers Energy-dispersive (ED) analyzers are heavily used in: – X-ray fluorescence (XRF), especially portable or small-footprint – Electron microprobe (SEM) The “spectrometer” is just a Si(Li) detector. – Si(Li) detectors are made of silicon doped with Li, usually cooled using LN2 or a refrigeration system Usually called lithium-drifted silicon, also drifted germanium. – The detector is polarized with a high voltage When x-ray photons hit the detector, electron-hole pairs are created that drift through the potential, creating a “pulse” that’s magnitude is directly proportional to the xray energy Energy-Dispersive Analyzers The Si(Li) detector: Energy-Dispersive Analyzers: Typical Spectra An ED X-ray spectrum from a Si(Li) detector, for qualitative analysis: J. I. Goldstein, D. E. Newbury, P. Echlin, D. C. Joy, A.D. Romig, Jr., C. E. Lyman, C. Fiori, and E. Lifshin , Scanning Electron Microscopy and X-Ray Microanalysis,” 2nd Edition, Plenum Press, 1992. Wavelength-Dispersive Analyzers General layout of a WD X-ray monochromator and detector: “Sample” (source of X-rays) Detector (pulse height detector) Reflection occurs when: n 2d sin sin n 2d Wavelength-dispersing crystal Total = 2 Wavelength-Dispersive Analyzers The Rowland design: Diagram from Strobel and Heineman, Chemical Instrumentation, A Systematic Approach, Wiley, 1989. Wavelength-Dispersive Analyzers: Typical Spectra WD offers much higher energy resolution than ED, better sensitivity, and better reproducibility (precision) for quantitative analyses Figures from McSwiggen and Associates, www.mcswiggen.com Comparison of WD and ED X-ray Detectors Most important advantages of WD: Higher resolution, sensitivity Most important advantages of ED: Cheaper, faster (except for multichannel WD) Other differences (more detailed comparison): Energy-Dispersive Wavelength-Dispersive Fast qualitative analysis Slow qualitative analysis Non-focusing spectrometer Focusing spectrometer Analyzes all elements at once Analyzes one/few element(s) at a time Low count rates (~2000 counts/sec) High count rates (~50000 counts/sec) Poor resolution (140-150 eV/channel) Good resolution (5 eV/channel) Limited detection limits (1% w/w) Good detection limits (0.01% w/w) Adequate quantitative analysis Excellent quantitative analysis (0.03%) Poor light element detection (typically down to boron with windowless designs) Excellent light element detection, including quantitative analysis down to beryllium Higher background (lowers S/N) Lower background (increases S/N) Less expensive (simpler) More expensive (complex) The future – CdTe and CdZnTe materials as ED detectors X-ray Fluorescence (XRF) Spectrometry Review of the principles: – if an X-ray photon (the primary X-ray) is absorbed by an atom, and it has enough energy, it can eject an electron, leaving a vacancy – A higher energy electron will drop down to replace it, emitting a “secondary” X-ray – The energy of the secondary X-ray (if it can be detected) is the difference of the binding energy of the two shells!!! XRF is a similar process to the “photoelectric effect” – where an x-ray is absorbed and transfers all of its energy to an electron Both ED and WD spectrometers are widely available X-ray Fluorescence X-ray Fluorescence (XRF) The XRF yield is actually influenced by the degree of Auger electron formation – Auger electrons predominate at lower Z XRF can be produced by: – – – – X-rays Alpha particles (APXS) Protons (PIXE) Electron beams (SEM electron microprobe) K number of K photons produced number of K shell vacancies created Auger 1 K XRF: Typical Spectra An ED XRF spectrum of a calibration standard: Advantages and Disadvantages of XRF Advantages: – Can be applied in-situ and nondestructively to analytes with little or no sample preparation – Speed – very fast – Good accuracy and precision Disadvantages: – Not as sensitive as UV/Vis methods for elemental analysis (only gets down to ppm level in some cases) – Auger process reduces sensitivity for lighter elements (Z < 23) – Windows and other spectrometer components can limit elements to those with atomic numbers greater than 5-6 (i.e. carbon) Philips PW2400 WDS Applications of XRF to Qualitative and Quantitative Analysis Matrix Effects – Fluorescent X-rays can be produced by both the analyte and the matrix Electronic materials – measurement of defects (elemental impurities) in silicon Machinery – analysis of metal composition, effects of machining, defects and abnormalities Ceramics – elemental composition and impurities Biological specimens and foods Petrochemicals – analysis of liquids, catalysts, etc… Example: Calcium quantitative analysis in calcium carbonate antacid tablets – Entire tablets can be analyzed in situ Hand-Held XRF Technology Miniaturized XRF technology applications are growing: – – – – Mining Geology Environmental analysis Alloy analysis http://www.spectroscopymag.com/spectroscopy/article/articleDetail.jsp?id=406625 Utilize lightweight x-ray tubes and Si PiN diode detector – No radioactive isotopes The Innov-X Systems “Alpha Series”, see http://www.innov-xsys.com Applications of Hand-Held XRF Technology Rapid, non-invasive XRF analysis of wood waste found in Hurricane Katrina debris for arsenic Wood contains chromated copper arsenate (CCA, now banned), which was used to pressure-treat lumber – Detection limit for As in low-density samples is 10-100 ppm – Using K and K lines at 10.54 and 11.73 keV B. Dubey, H. M. Solo-Gabriele, and T. G. Townsend, “Quantities of Arsenic-Treated Wood in Demolition Debris Generated by Hurricane Katrina”, Environ. Sci. Technol. 41(5) 1533–1536 (2007). Scanning Electron Microscopy and X-ray Microanalysis A scanning electron microscope is a popular excitation source for X-ray emission – Electrons (5 keV – 30 keV) hit a sample. – They penetrate about 1 um – They knock loose K and L shell electrons X-rays are emitted as higher energy electrons drop down to fill the “hole” J. I. Goldstein, D. E. Newbury, P. Echlin, D. C. Joy, A.D. Romig, Jr., C. E. Lyman, C. Fiori, and E. Lifshin , Scanning Electron Microscopy and X-Ray Microanalysis,” 2nd Edition, Plenum Press, 1992. Electron-Induced X-ray Emission X-ray Emission in Electron Microscopy X-ray Emission is just one of a multitude of processes that can occur when electrons hit a target In an SEM/TEM/STEM, the following are possible: – X-ray emission spectrometry with mapping – Formation of images from backscattered electrons – Diffractometric analysis Will be discussed in the “Surface Analysis” Lecture PIXE: X-ray Emission: PIXE particle (proton) induced x-ray emission Diagram is from the PIXE system at Harvard: requires a particle accelerator (5-10 meters long) PIXE is heavily used in art conservation and archaeology Diagram of PIXE Instrument from www.mrsec.harvard.edu (2006) X-ray Emission: PIXE PIXE: Just like electroninduced x-ray emission, only more efficient – Less damaging to the sample but more sensitive – Less charging than electrons – Less lateral deflection (protons are not multiply scattered like e-) PIXE images from www.ipp.phys.ethz.ch and www.tiara.taka.jaeri.go.jp (2006) X-ray Emission: APXS APXS: alpha particle x-ray spectrometry Alpha particles better for exciting light elements: – Na, Mg, Al, Si X-rays better in exciting heavier elements – Fe, Co, Ni Relative effectiveness crosses over at chromium APXS – a compact ED spectrometer for light-medium elements with a radioactive curium-244 source Images from www.nasa.gov (2006) X-ray Emission: APXS APXS spectra from Mars: easy detection from sodium to iron Images from www.nasa.gov (2006) X-ray Absorption X-ray absorption is used for totally different applications that X-ray fluorescence and emission. Beer-Lambert law: ln P0 x P where is the linear absorption coefficient (depends on the element and #of atoms): Z 4 AE 3 (E is the energy of the x-rays, A is the atomic mass and Z is the atomic number). Also: ln P0 P P0 M x P where M is the mass absorption coefficient, which is independent of the element’s state and is the density x X-ray Absorption Why do X-ray and atomic/molecular UV-Vis absorption spectra look so different, with all that the two techniques have in common? – Atomic absorption/UV-Vis spectra have peaks – X-ray absorption spectra have edges Answer: the ionization! – Optical AA has a peak with a narrow bandwidth because an outer shell electron is excited to a higher energy level – a discrete quantum process – X-ray absorption is caused by photoelectron ionization – not as discrete of a process – since energy in excess of that required for ionization appears as kinetic energy of the photoelectron. X-ray Absorption Fine Structure (XAFS) X-ray absorption fine structure (XAFS) refers to the details of how x-rays are absorbed by an atom at energies near and above the core-level binding energies of that atom. Specifically, XAFS is the modulation of an atom’s x-ray absorption probability due to the chemical and physical state of the atom. XAFS spectra are sensitive to the oxidation state, coordination chemistry, and the distances, coordination number and species of the atoms immediately surrounding the atom of interest. XAFS needs an intense, energy-tunable source of X-rays (a synchrotron). X-ray Absorption Fine Structure (XAFS) Two regions of the XAFS spectrum: – EXAFS (extended x-ray absorption fine structure): Sensitive to distances, coordination number, and identity of surrounding atoms – XANES (X-ray absorption near edge spectroscopy): Sensitive to oxidation state and coordination (e.g. tetrahedral vs. octahedral coordination of an atom). Diagram from M. Newville, “Fundamentals of XAFS”, University of Chicago, 2003. EXAFS Slide from M. Newville, “Fundamentals of XAFS”, University of Chicago, 2003. EXAFS Slide from M. Newville, “Fundamentals of XAFS”, University of Chicago, 2003. XANES XANES – often empirically interpreted – see new ref Rehr, J. J., Ankudinov, A. L., Progress in the theory and interpretation of XANES Coordination Chemistry Reviews, Jan 2005 Diagram from M. Newville, “Fundamentals of XAFS”, University of Chicago, 2003. X-ray Photoelectron Spectroscopy and Related Techniques Scanning Auger, XPS, UPS, ECSA, and more… All are surface analysis methods and will be discussed during the “Microscopy and Surface Analysis” lecture. Diagram from Charles Evans and Associates website (http://www.cea.com) http://www.cea.com/cai/augtheo/caiatheo.htm Homework Problems From Chapter 12 of Skoog et al.: 12-2 12-9 Further Reading I. Szaloki, et al., “X-ray Spectrometry”, Anal. Chem., 2002, 74, 2895-2918.