Helvetica bold 30 pts two lines
advertisement

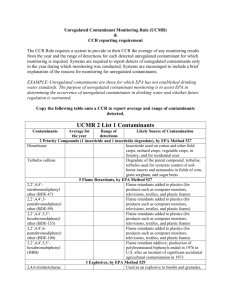
UCMR3 Sampling Workshop: What You Need to Know Presented by: Joseph Mattheis Life and Health Sciences Water Quality Account Executive UL and the UL logo are trademarks of UL LLC © 2012 Agenda About UL UCMR 3 Overview UCMR 3 Methods UCMR 3 Sample Collection UCMR 3 Costs Questions 2 UL at a Glance: Standards Development, Testing, Certification, Knowledge Services UL founded by William Henry Merrill following 1893 Chicago World’s Fair 1897 Takes name of Underwriters’ Laboratories, Inc. First international office opened in London 1903 1958 1901 1894 First published list of approved electrical devices UL/CCIC agreement for inspections 1916 First UL standard published STR QA acquisition 1988 1980 Fire protection lab built 2011 Hong Kong and Taiwan offices open 118 year history 105 facilities globally 60,000+ customers in 102 countries 10,000+ employees 23 billion UL marks appear on 72,000 products 2.0 billion consumers reached Mission: Promote safe living and working environments for people around the world 3 About UL Safe Living & Working Environments Facilitating Global Trade Trusted Source Serving the Public Safety Mission Globally • Our mission enables industry and geographic growth • Our global footprint and scale support our customers • Our legacy, integrity and knowledge platform provide unparalleled information to customers 4 UL Business Units Product Safety Encompasses UL’s traditional testing and certification business; helping manufacturers bring safer products to global markets faster Verification Services Provides performance / verification testing and inspection services for manufacturers and retailers Life & Health Focuses on human safety in the medical, food, and water industries Knowledge Services Offers training, education, and technical expertise in product safety and related areas Environment Provides environmental claims validation and certification to help guide industries, governments, and consumers with sustainability and environmental product-related decisions 5 Drinking Water Analytical Services Analytical Expertise At our state-of-the-art 70,000 square foot facility, UL performs virtually all analyses required under the Safe Drinking Water Act (SDWA) including: • Dioxin • Disinfection byproducts • Metals • Microbial contaminants • Organic & inorganic constituents • Radiological parameters UCMR 3 Overview • Purpose • Reporting • Timeline • PWSs • Applicability • Monitoring • Elements • Dates • Data • Lists • Contacts • Contaminants • Methods • Sampling Frequency • Sampling Instructions • Sampling Locations 8 Purpose of UCMR 3 • 1996 Amendments to SDWA require EPA to monitor unregulated contaminants • Specifies frequency of every 5 years • Data generated allow for Public exposure assessment to unregulated contaminants of concern • Data provide the basis for regulatory decisions – which contaminants to regulate 9 UCMR 3 Time Frame • December 21, 2010 – applicability date • State monitoring plans developed (including national representative sample based on SDWIS/Fed population) • March 3, 2011 – proposed rule published • Laboratory approval program begins • May 2, 2012 – final rule published • States/EPA begin to inform PWSs and finalize monitoring plans 10 UCMR 3 Timeline 2012 2013 2014 2015 2016 Pre-monitoring Implementation • Lab Approval • SDWARS Registration - Inventory - Schedule Sampling Period One consecutive 12-month period during January 2013 – December 2015 (monitoring can span more than 1 calendar year, as long as conducted in a consecutive 12- month period). Complete Reporting to SDWARS • Review Data • Post to NCOD 11 UCMR 3 Applicability – Who is Required To Test? Assessment Monitoring (List 1 Contaminants) • All CWS & NTNCWS serving populations >10,000 (~4,200) plus 800 randomly selected serving populations ≤ 10,000 Screening Survey (List 2 Contaminants) • All CWS & NTNCWS serving populations >100,000 (~410) ; ~320 randomly selected serving populations 10,001 to 100,000 and 480 randomly selected serving populations ≤ 10,000 Pre-Screen Testing (List 3 Contaminants) • 800 Randomly selected systems serving populations ≤ 1,000 12 UCMR 3 Monitoring Lists Assessment Monitoring – List 1 Contaminants • EPA Method 524.3 Volatile Organic Compounds - 1,2,3-trichloropropane - 1,3-butadiene - chloromethane - 1,1-dichloroethane - bromomethane - chlorodifluoromethane - bromochloromethane 13 UCMR 3 Monitoring Lists Assessment Monitoring – List 1 Contaminants (cont.) • EPA Method 537 - Perfluorinated Compounds - perfluorooctane sulfonic acid (PFOS) - perfluorooctanoic acid (PFOA) - perfluorononanoic acid (PFNA) - perfluorohexane sulfonic acid (PFHxS) - perfluorophtanoic acid (PFHpA) - perfluorobutaneseulfonic acid (PFBS) • EPA Method 522 - Synthetic Organic Compounds - 1,4-Dioxane 14 UCMR 3 Monitoring Lists Assessment Monitoring – List 1 Contaminants (cont.) • EPA Method 300.1 - Chlorate - chlorate • EPA Method 200.8 - Metals (alternate methods also available) - vanadium - molybdenum - cobalt - strontium - chromium • EPA Method 218.7 - Chromium6 (Cr6) - chromium-6 15 UCMR 3 Monitoring Lists Screening Survey – List 2 Contaminants • EPA 539 - Hormones • 17-β-estradiol • 17-α-ethynylestradiol (ethinyl estradiol) • 16-α-hydroxyestradiol (estriol) • equilin • esterone • testosterone • 4-androstene-3,17-dione 16 UCMR 3 Monitoring Lists Pre-Screen Testing – List 3 Contaminants • Cell Culture or QPCR - enterovirus • QPCR - norovirus • Various Methods - Microbiological Indicators - total coliforms - E. coli - enterococci - bateriophage - aerobic spores EPA will collect the samples from List 3 sampling locations and will pay for all analytical and shipping costs associated with viruses and indicators for these small systems (≤ 1,000). 17 UCMR 3 Monitoring Frequency PWSs must monitor during a consecutive 12-month period between 2013 - 2015 • Number of times a PWS samples is directly related to the sample point source - Surface Water and Ground Water under the Direct Influence of Surface Water – must monitor quarterly during their 12-month schedule (sampling events three months apart) - Ground Water – must monitor twice during their 12-month schedule (sampling events five to seven months apart) • EPA established a monitoring schedule for all PWSs - PWSs can change their pre-determined schedule in SDWARS by November 29, 2012, independently, or after that date with EPA permission 18 UCMR 3 Monitoring Locations All samples for UCMR 3 contaminants will be collected at the Entry Point to the Distribution System (EP or EPTDS); in addition three methods will also be collected at the Distribution System Maximum Residence Time (DSMRT) List 1: Assessment Monitoring Contaminants Sampling Location Type Contaminant Type EPA 524.3: EPA 537: EPA 522: EPA 300.1: EPA 200.8: EPA 218.7: Volatile Organic Compounds Perfluorinated Compounds Synthetic Organic Compounds Chlorate Metals Chromium-6 EPTDS DSMRT x x x x x x x x x List 2: Screening Survey Contaminants Contaminant Type Sampling Location Type EPTDS EPA 539: Hormones DSMRT x 19 UCMR 3 Monitoring Locations – Field Blanks Due to susceptibility to outside source contamination, UCMR3 requires field blanks to be processed, extracted and analyzed for three of the List 1 Methods and the List 2 Method if there is a contaminant detected in the associated field sample. Field Blanks will be included with the following methods: List 1 List 2 • EPA Method 524.3 • EPA Method 539 • Collected at the EPTDS - Collected at the EPTDS • EPA Method 537 • Collected at the EPTDS • EPA 200.8 • Collected at the EPTDS & at the DSMRT 20 UCMR 3 Monitoring and Reporting Small PWSs serving 10,000 or fewer people are not responsible for the costs associated with the analysis • EPA coordinates sample analyses with contracted laboratories • Laboratories submit the data directly to the EPA • EPA examines the quality control results and generates reports • EPA collects samples for only List 3 contaminants • PWS reviews and can act upon data in SDWARS 3 21 UCMR 3 Monitoring and Reporting Large PWSs serving more than 10,000 people are responsible for the costs associated with the analyses • PWS coordinates sample analyses with an approved laboratory, and that lab sends the data to the Safe Drinking Water Accession and Review System (SDWARS 3) • http://cdx.epa.gov/epa_home.asp • PWS reviews and can act upon data in SDWARS 3 22 UCMR 3 Reporting Elements UCMR 3 Reporting Elements Public Water System Identification Code Disinfection Type Sample Analysis Type Public Water System Facility Identification Code Sample Collection Date Analytical Results - Sign Water Source Type Sample Identification Code Analytical Results - Value Sampling Point Identification Code Contaminant Laboratory Identification Code Sampling Point Type Identification Code Analytical Method Code Sample Event Code As a one-time reporting requirement, PWSs must report the U.S. Postal Service Zip Code(s) for all areas being served water by that PWS. 23 UCMR 3 Reporting Elements All of the disinfectants that have been added to the water being sampled must be reported by systems for each sampling point with possible choices being: • CLGA – Gaseous chlorine • CLOF – Offsite Generated Hypochlorite (stored as liquid) • CLON – Onsite Generated Hypochlorite (no storage) • GAGC – Chloramine (formed from gaseous chlorine) • CAOF – Chloramine (formed from offsite hypochlorite) • CAON – Chloramine (formed from onsite hypochlorite) • CLDO – Chlorine Dioxide • OZON – Ozone • ULVL – Ultraviolet Light • OTHD – All other Types of Disinfectant • NODU – No Disinfectant Used 24 UCMR 3 Key Dates – November 29, 2012 Systems must submit contact information to SDWARS. • Subsequent changes must be submitted within 30 days of the change occurring Systems can change their monitoring schedule, and review and edit if necessary, inventory information for sampling locations. • After November 29th, systems must request a change (with an explanation) and obtain EPA approval of the change 25 UCMR 3 Data Reporting Samples must be analyzed by EPA-approved laboratories. • EPA-approved laboratories will be listed on the UCMR website at: http://water.epa.gov/lawsregs/rulesregs/sdwa/ucmr/ucmr3/laboratori es.cfm • Within 120 days of sample collection - Laboratories post data to SDWARS • Within 60 days of lab posting data - PWS reviews and approves the data. If the PWS has not taken action after 60 days, the data are considered approved and ready for state and EPA review 26 EPA Use of UCMR 3 Data • Updated quarterly by EPA and posted in the National Contaminant Database (NCOD) • www.epa/safewater/databases/ncod/index.html • Data will continue to be added and may be corrected upon further review • Use caution when interpreting the data before the dataset is complete • UCMR 3 is one of the primary sources of occurrence and exposure information the agency uses to develop regulatory decisions for contaminants of concern 27 UCMR 3 Contacts • UCMR Message Center: (800) 949-1581 • This number may change later this year, but as of Sept 7th is still in use • Safe Drinking Water Hotline: (800) 426-4791 • CDX/SDWARS Help Desk: (888) 890-1995 • UCMR Sampling Coordinator or Laboratory Approval Coordinator UCMR_Sampling_Coordinator@epa.gov USEPA Technical Support Center 26 West Martin Luther King Drive (MS 140) Cincinnati, OH 45268 28 UCMR 3 EPA Online Resources • UCMR 3 EPA Website • http://water.epa.gov/lawsregs/rulesregs/sdwa/ucmr/ucmr3/ - Home - Basic Information - Methods & Contaminants - Laboratories - Reporting 29 UCMR 3 Methods Assessment Monitoring (List 1 Contaminants) primarily utilize common analytical method technologies used by drinking water laboratories. • 524.3 GC/MS • 522 Quadrupole GC/MS • 300.1 Ion-Chromatography • 218.7 Ion-Chromatography • 200.8 ICP-MS • 537 LC/MS/MS 30 UCMR 3 Methods Screening Survey (List 2 Contaminants) primarily uses common analytical method technologies used by drinking water laboratories. • 539 LC-ESI-MS/MS Pre-Screen Testing (List 3 Contaminants) uses newer method technologies not as commonly used by drinking water laboratories. For UCMR 3 these PWSs are ground water systems that: • Serve less than 1,000 people • Do not disinfect • Are located in areas of karst or fractured bedrock 31 UCMR 3 Sampling Information Partnering with your selected laboratory is crucial to your success! • Complete and Accurate detail on the Chain of Custody and bottle labels is critical for successful data uploads to SDWARS • Following the collection schedule • Understand the collection and preservation instructions (Method 522 requires a 2 step preservation process) • Be certain that both you and your selected laboratory clearly understand the field blank protocols 32 UCMR 3 Sampling Information Properly handling Field Blanks is critical to avoid costly and time consuming recollections. • Four Methods require field blanks with each sample • 524.3 (in duplicate) • 200.8 (at both the EPTDS and the DSMRT) • 537 • 539 • Collection protocols differ depending on the method • Any field sample with a detect requires analysis of the associated field blank • Field Samples with a detect greater than >1/3 MRL requires re-collection 33 UCMR 3 Sampling Information Both the sample collector and the primary PWS contact (if not the same person) need to know and understand the Field Blank protocols. • 524.3 (EPTDS) • Pre-preserved vials filled with reagent water shipped with collection containers • DO NOT open in the field • 200.8 (EPTDS and DSMRT) • Bottle filled with reagent water shipped with collection containers • At the collection site, OPEN the Field Blank, recap, and return with the field samples to the laboratory • 537 and 539 (EPTDS) • Pre-preserved bottles with reagent water as well as a empty bottle shipped with collection containers • At the collection site, transfer the preserved reagent water into the corresponding empty container and return with the field samples to the laboratory 34 Sampling Instructions Before Sampling • Read all instructions thoroughly as there are multiple sampling procedures required. • Remove refrigerant packs from shipping container and place in freezer. The packs must be frozen before returning samples to the laboratory. • Plan to have samples collected just prior to the normal pick-up time of your overnight carrier. 35 Sampling Instructions Sampling Steps 1.If your sampling point has a faucet with an aerator, it must be removed prior to collection of the samples. 2.Flush the cold water sampling line approximately 10 minutes immediately prior to sampling. 3.Do not touch inside the cap or around the edge of the bottle. 4.Slow the water stream before collection. 5.Refer to the method-specific sampling instructions described below. 6.Indicate sampling date, time, site, and name of sampler on both the bottle labels and the enclosed Chain of Custody. 7.Information on the Chain of Custody and labels must match and be complete. 8.Caution: Bottles and vials may contain chemical preservatives. Avoid skin contact! 36 Method-Specific Sampling Instructions UCMR 3 Volatiles – EPA 524.3: • Fill three vials so that no air remains when capped. • Do not overfill. Do not flush away the preservative. • After tightening the cap, the vial should be inverted and tapped to check for air bubbles. • If bubbles are present, do not empty the vial. Slowly add several additional drops of water until all air is eliminated. 3 Amber glass 40 ml vials with Teflonfaced silicone septa in polypropylene caps. Storage temp: <= 10 C first 48 hrs., <= 6 C after 48 hrs. Hold Time: 14 days Preservative: 20-30 mg ascorbic acid + 180-220 mg maleic acid 37 UCMR 3 Volatiles – EPA 524.3 Field Reagent Blanks: • The bottles with the blue label are Field Reagent Blanks (FRB). • DO NOT OPEN in the field. These field blanks must remain sealed. • Return with field sample. UCMR 3- Method 524.3 Field Reagent Blanks *DO NOT OPEN 38 UCMR 3 Hexavalent Chromium – EPA 218.7: • Remove cap, fill the sample bottle to the neck, then replace cap and tighten. Do not overfill. Do not flush away the preservative. 1 High density polyethylene (HDPE) 120 ml bottle, collect 100 ml of sample. Storage temp: <= 10 C first 48 hrs., <= 6 C after 48 hrs. Hold Time: 14 days Preservative: 68.2-73.8 mg solid Na2CO3/NaHCO3/(NH4)2SO4 buffer mixture 39 UCMR 3 Chlorate – EPA 300.1 Is chlorine dioxide used as part of your disinfection treatment process? No Yes Use Standard Sampling Procedures Use Sparging Procedure Prior to Sampling 40 UCMR 3 Chlorate – EPA 300.1: Standard Sampling Procedure • Remove cap, fill the sample bottle to the neck • Replace cap and tighten. Do not overfill. • Do not flush away the preservative. 1 Plastic 120 ml bottle Storage temp: Ambient Hold Time: 28 days Preservative: 0.12 ml 5% EDA 41 UCMR 3 Chlorate – EPA 300.1: Sample Sparging Procedure for Systems Using Chlorine Dioxide 120 mL plastic labeled sample bottle (prepreserved) plus a 250 mL plastic unlabeled sparging bottle 42 UCMR 3 Chlorate – EPA 300.1: Sample Sparging Procedure for Systems Using Chlorine Dioxide (cont.) Disposable glass Pasteur pipette with PVC tubing. Sparging gas should bean inert gas such as a lecture bottle of nitrogen or helium fitted with a regulator. 43 UCMR 3 Chlorate – EPA 300.1: Sample Sparging Procedure for Systems Using Chlorine Dioxide (cont.) • Remove cap from the unlabeled 250 mL plastic bottle and fill approximately ¾ full with sample • Insert the pipette and adjust gas flow to produce a steady flow of bubbles • Sparge the sample for approximately 10-15 minutes. The chlorine dioxide should be effectively removed from the sample. 44 UCMR 3 Chlorate – EPA 300.1: Sample Sparging Procedure for Systems Using Chlorine Dioxide (cont.) • Transfer the sparged sample into the EDA preserved 120 mL bottle filling to the neck of the bottle. • Replace the cap on the 120 mL bottle and tighten. • Dispose of any extra sparged sample and its container. 45 UCMR 3 Metals – EPA 200.8: • Remove cap, fill the sample bottle to the neck, then replace cap and tighten. 250 mL Plastic Bottle Hold Time: 14 days for unpreserved samples, 6 months for samples preserved within 14 days 46 UCMR 3 Metals – EPA 200.8 Field Trip Blank: • The bottle with the blue label is a Field Trip Blank (FTB). • Break the custody seal and OPEN the bottle. • Recap the bottle at the sampling site after collecting field samples. • Return with the field sample. • The custody seal must be broken. UCMR 3-200.8 Field Trip Blank *Open at the sampling site *Replace cap and tighten, safety seal must be broken 47 UCMR 3-1,4-Dioxane – EPA 522: • Remove cap, fill the sample bottle to the neck, then replace cap and tighten. • Do not overfill. Do not flush away the preservative. 3 Amber Glass 250 ml bottles with Teflon lined caps pre-preserved with 20 +/- 2 mg Na2SO3 1 small vial of 250 +/- 25 mg NaHSO4 to be added at sampling Storage temp: <= 10°C first 48 hrs., <= 6°C after 48 hrs. Do not freeze. Hold Time: 28 days • Cap the bottle and shake vigorously to dissolve the de-chlorinating agent sodium sulfite. • Uncap the bottle. Add entire contents of vial of sodium bisulfate preservative into the sample. • Cap the bottle and shake vigorously to dissolve the preservative. 48 UCMR 3 PFCs – EPA 537: • Nitrile gloves (provided) MUST be worn during the sampling steps. • Remove cap, fill the sample bottle to the raised line on the bottle. • Replace cap and tighten. • Do not overfill. Do not flush away the preservative. 3 Plastic 250 ml bottles with polypropylene caps. Storage temp: <= 10 C first 48 hrs., <= 6 C after 48 hrs. Hold Time: 14 days Preservative: 1.2-1.3 g Trizma Preset 49 UCMR 3 PFCs – EPA 537 Field Trip Blank: • The bottle with the blue label is a Field Trip Blank (FTB). • Break the custody seal, open the bottle, and then pour the Field Trip Blank Water into the empty bottle with a blue label. • Return with the field sample. UCMR 3-537 Field Trip Blank *Transfer the filled bottle into the empty FTB bottle 50 UCMR 3 Hormones – EPA 539: • Nitrile gloves (provided) MUST be worn during the sampling steps. • Remove cap, fill the sample bottle to the neck, then replace cap and tighten. • Do not overfill. Do not flush away the preservative. 3 Amber Glass 1 L bottles with Teflon lined caps. Storage temp: <= 10 C first 48 hrs., <= 6 C after 48 hrs. Hold Time: 28 days Preservative: 75-85 mg sodium thiosulfate + 60-70 mg sodium omadine 51 UCMR 3 Hormones – EPA 539 Field Trip Blank: • The bottle with the blue label is a Field Trip Blank (FTB). • Break the custody seal, open the bottle, and then pour the Field Trip Blank into the empty bottle with a blue label. • Return with the field sample. UCMR 3-539 Field Trip Blank *Transfer the filled bottle into the empty FTB bottle 52 Sample Shipping Instructions Shipping Instructions: • Place frozen refrigerant packs, bags of wet ice, samples and Chain of Custody into shipping container and return to the laboratory immediately after collection. • Sample bottles must be hand delivered or sent by an overnight carrier. • Laboratory must be notified prior to shipment of samples for Saturday delivery. 53 UCMR 3 Distribution Kit 54 Chain Of Custody - DSMRT 55 UCMR 3 List 1 Kit 56 Chain Of Custody – EPTDS List 1 57 UCMR 3 List 1 + List 2 Kit 58 Chain Of Custody – EPTDS List 1 and 2 59 UCMR 3 Collection Video 60 Why is UCMR 3 Testing Expensive? EPA made assumptions that laboratories would take steps with all field blanks to ensure that if analysis of any of the blanks becomes necessary, required holding times would not be exceeded. These assumptions include: • All 524.3 field blanks would be analyzed • All 537 and 539 blanks would be extracted • EPA’s conservative assumption that all 537 and 539 Field Blanks will be extracted • Extraction is only part of the process, it only covers a portion of the total cost if a sample does end up requiring analysis such as • Costs associated with additional analytical batches • Additional staff salaries for analysis, peer review, client notifications, report generation, and reporting to the client and SDWARS 61 Why is UCMR 3 Testing Expensive? (cont.) Parameter CAS # Number of Finished Water samples Analyzed Number of Detects > UCMR3 MRL UCMR3 MRL % w/ Detects >MRL Chromium 7440-47-3 16269 12441 0.2 ug/L 76% Cobalt 7440-48-4 7949 516 1.0 ug/L 6% Molybdenum 7439-98-7 8232 4221 1.0 ug/L 51% Strontium 7440-24-6 9427 9049 0.3 ug/L 96% Vanadium 7440-62-2 8809 4996 0.2 ug/L 57% • 200.8 Field Blanks were NOT mentioned in EPA’s cost assumptions. • Based on a high rate of occurrence as illustrated in the table above, expect field blanks for 200.8 to require analysis. • A Field Blank that requires analysis is not different than a field sample and cost the laboratory the same amount to analyze. • Assuming a PWS has just 1 EPTDS and 1 DSMRT, this makes four analyses 62 UCMR 3 Costs - Budgeting & Planning There is no doubt that UCMR 3 is an inexpensive regulation. There are, however, a few things that you can do to spread the cost out and minimize the potential for additional costs. • Adjust your schedule in SDWARS so collections fall over 2 fiscal years instead of 1 • 12 consecutive months does not mean those twelve months must be in the same calendar year • If you are required to perform Assessment Monitoring (List 1), a SW supply can collect the last two quarters of one year and the first two of the next year • If you are required to perform Screening Survey monitoring, you could collect all of your List 1 samples in one year and all of your List 2 samples in the next year • Memorize the Field Blank protocols, this will reduce the likelihood of contamination during collection and potentially avoid additional laboratory fees (some may bill all or a percentage for sample and Field Blank analysis if it is invalid due to contamination during collection) 63 Questions?