Pregnancy Testing - Austin Community College
advertisement

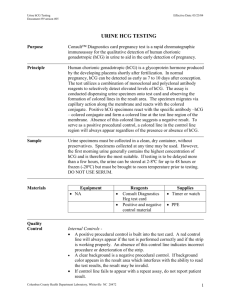
Pregnancy Testing Terry Kotrla, MS, MT(ASCP)BB Austin Community College Introduction • Most pregnancy tests used today, whether a home urine test, a physician's office urine or blood test, or a clinical laboratory blood test are "sandwich assays". • Sandwich assays use two or more animal antibodies raised against different sites on Human Chorionic Gonadotropin (hCG). • Usually a mouse monoclonal antibody against one site on the hCG molecule, and a mouse monoclonal, or a sheep, rabbit or a goat polyclonal antibody against a second distant site on the hCG molecule. Simple to Perform But understanding the concepts of the principle may be challenging. SUMMARY AND EXPLANATION OF THE TEST • Human chorionic gonadotropin (hCG) is a glycopeptide hormone produced by the placenta during pregnancy. • The appearance and rapid rise in the concentration of hCG in the woman's urine makes it a good pregnancy marker. • Usually, concentration of hCG in urine is at least 25 mIU/ml as early as seven to ten days after conception. • The concentration increases steadily and reaches its maximum between the eighth and eleventh weeks of pregnancy. Fertilization PRINCIPLE OF THE TEST • • • • • • • The pregnancy testing device contains a unique set of dye-conjugated and immobilized antibodies used to produce a distinctive visual pattern indicating elevated concentration of hCG (=25 mIU/ml) in the test sample. One antibody, the capture antibody, is in a solid phase permanently attached to a tube, plate, membrane, or bead. Conjugate pad contains the label reagent, i.e. antibody labeled with either red, gold or blue latex particles. Sample is applied and dissolves the label mixture and migrates to the zone of immobilized antibody lines. If hCG is present, labeled antibody-dye conjugate binds it, forming an antibody-antigen complex. Positive, that is hCG containing, sample causes the formation of a colored test line, which indicates a positive test result. As the reaction mixture continues to flow along the test membrane, the complex binds to the anti-hCG antibody in the test zone of the membrane, and produces a color band. Unbound conjugate binds to the reagents immobilized in the control zone producing a color band, demonstrating proper performance of the test. Figure 1 • Device with solid phase capture antibody to one site on hCG, and liquid phase tracer antibody (label shown by red star) to second or distant site on hCG. In this way the label becomes immobilized. Figure 2 • Serum or urine containing hCG (shown as ab) added to device. The hCG forms a sandwich or bridge between capture and tracer antibody. After a short incubation period the hCG binds both the solid phase and liquid phase antibodies linking them. Figure 3 • Excess tracer antibody is washed away. Amount of label or tracer (red star) is measured. This is proportional to amount of hCG. Illustration of Principle TEST PROCEDURE • NOTE: Bring test components and specimens to room temperature prior to testing. • Remove a Testing Device from the foil pouch by tearing at the "notch" and place it on a level surface. • Holding a Sample Dropper vertically, add exactly four drops of the urine specimen to the sample well. NOTE: Picture shows incorrect orientation of dropper to test area, must be completely vertical to ensure adequate sample. • Read results at time indicated in procedure. Interpretation • If two color bands are visible the test is positive. • The presence of a Control Band only indicates a negative test. Results Function of the Control Band • The Control Band is used as a reference and built in quality control check. • If the Test Band is darker or similar to the Control band, the test result is considered positive. • The Control Band is used for procedural control to check whether the test reagents are working properly and that a sufficient amount of urine sample has been applied to the test area. Invalid Tests • If, after performing the test, no purple color band is visible anywhere within the Results Window, the result is considered invalid. • If a color appears in the test area but NO color appears in the control area, the test is invalid. Ovulation Test Another Example of Results Causes of Invalid Results • The directions may not have been followed correctly. • Inadequate amount of sample has been exposed to the test system. • The test may have deteriorated. PRECAUTIONS • Do not use test kit components after the expiration dates. • Dispose of all used test components in a proper biohazard container. • If specimens or test components have been stored in a refrigerator, allow them to warm to room temperature before performing the test. • Human specimens should be handled as if capable of transmitting infectious agents. Limitations of the Procedure • • • • • • • Besides pregnancy, elevated concentrations of hCG may be found in patients with both gestational and non-gestational trophoblastic diseases. These conditions should be ruled out in the interpretation of hCG levels to establish a diagnosis of pregnancy. A low incidence of false results can occur. Consult with a physician if unexpected or inconsistent results. A normal pregnancy cannot be distinguished from an ectopic pregnancy based on hCG levels alone. A spontaneous miscarriage may cause confusion in interpreting the test results. A definitive diagnosis should not be based on the results of a single test, but should only be made by the physician after all clinical and laboratory findings have been evaluated. A negative result from a specimen collected from a woman in very early pregnancy may be due to an unusually low concentration of hCG. In such cases, the test should be repeated on a fresh specimen obtained approximately two days later. A urine sample may be too diluted and thus may not contain a representative concentration of hCG. If a negative result is obtained with a urine specimen and pregnancy is still suspected, obtain a first morning urine specimen and re-test. Ectopic Pregnancy • • This unfortunate patient came in with a late cycle. The pregnancy test was positive but we couldn't see a pregnancy on her ultrasound scan. We suspected an ectopic and this is what we found during laparoscopy. The pregnancy was lodged in the middle of her left tube. A small slit was made over this and the pregnancy was removed without removing her tube. Choriocarcinoma • Choriocarcinoma is a highly malignant germ cell tumour which usually follows an abnormal pregnancy with a hydatidiform mole. It may also occur after a spontaneous abortion, and rarely, may follow a normal pregnancy. The tumour metastasizes early, by means of vascular invasion and blood spread. Picture of liver. Choriocarcinoma of Testes Hydatiform Mole • Hydatidiform mole is a tumor of the placenta that is usually benign. It develops from placental tissue during an early pregnancy in which the embryo fails to develop normally. The tumor consists of many small vesicles (sacs) and resembles a large cluster of grapes. Although the condition is fairly rare in the USA, it is common in the Orient and other parts of the world. In the United States, molar pregnancy occurs in 1 of every 1,000-1,200 pregnancies. Seminoma • 50% of all testicular tumors. • Human chorionic gonadotropin-beta is a better marker than hCG. For earlier detection of recurrence, both markers should be examined. SPECIMEN COLLECTION AND STORAGE • First morning urine usually contains the highest concentration of hCG and is therefore the best sample when performing the urine test. However, randomly collected urine specimens may be used. • Collect a urine specimen in a clean glass, plastic, or wax coated container. Do not use preservatives. • If the test is not run immediately following collection of the sample specimen, but is to be run within 48 hours following collection, the specimen should be refrigerated (2-8°C), and brought back to room temperature (1528°C) before testing. • If testing is delayed more than forty-eight hours, the specimen should be frozen. A frozen specimen should not be used if stored more than two weeks. • Prior to testing, the frozen specimen must be completely thawed, thoroughly mixed, and brought to room temperature. QUALITY CONTROL • The use of controls is recommended to verify proper kit performance. • Quality control reagents (positive and negative) should be tested according to quality control requirements established by the testing laboratory. • Use controls in the same procedure as specimens. • This may be required with each test or only when a new lot number is being put into use. Reference • • http://www.saveontests.com/pregnancy_test_strip_instructions.htm http://www.ovulation-calculator.com/pregnancy-tests/pregnancy-tests.htm • http://sunflower.bio.indiana.edu/~aberndts/Z566/lab1images.html fertilization • http://www.healthsentinel.co.uk/professional/professional-pregnancy-test-info.htm • http://www.docsun.com/default.asp?id=26 – tubal pregnancy • http://www.moondragon.org/obgyn/pregnancy/molar.html • http://www.nlm.nih.gov/medlineplus/ency/article/001288.htm seminoma • http://www-medlib.med.utah.edu/WebPath/TUTORIAL/PRENATAL/PRENATAL.html Some pictures very graphic