Practice Quiz 1 Answer Key
advertisement
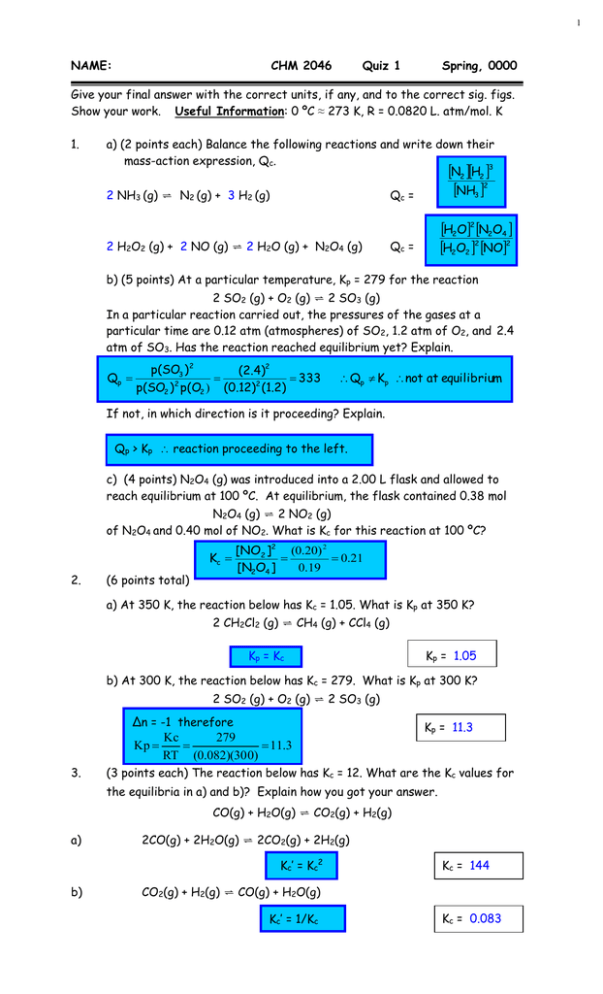
1 NAME: CHM 2046 Quiz 1 Spring, 0000 Give your final answer with the correct units, if any, and to the correct sig. figs. Show your work. Useful Information: 0 ºC ≈ 273 K, R = 0.0820 L. atm/mol. K 1. a) (2 points each) Balance the following reactions and write down their mass-action expression, Qc. N2 H2 3 2 NH3 (g) ⇌ N2 (g) + 3 H2 (g) NH3 2 Qc = 2 H2O2 (g) + 2 NO (g) ⇌ 2 H2O (g) + N2O4 (g) H2O2 N2O4 H2O2 2 NO2 Qc = b) (5 points) At a particular temperature, Kp = 279 for the reaction 2 SO2 (g) + O2 (g) ⇌ 2 SO3 (g) In a particular reaction carried out, the pressures of the gases at a particular time are 0.12 atm (atmospheres) of SO2, 1.2 atm of O2, and 2.4 atm of SO3. Has the reaction reached equilibrium yet? Explain. Qp p(SO3 ) 2 (2.4)2 333 p(SO2 ) 2 p(O2 ) (0.12)2 (1.2) Qp Kp not at equilibrium If not, in which direction is it proceeding? Explain. Qp > Kp reaction proceeding to the left. c) (4 points) N2O4 (g) was introduced into a 2.00 L flask and allowed to reach equilibrium at 100 ºC. At equilibrium, the flask contained 0.38 mol N2O4 (g) ⇌ 2 NO2 (g) of N2O4 and 0.40 mol of NO2. What is Kc for this reaction at 100 ºC? Kc 2. (6 points total) [NO2 ]2 (0.20) 2 0.21 [N2O4 ] 0.19 a) At 350 K, the reaction below has Kc = 1.05. What is Kp at 350 K? 2 CH2Cl2 (g) ⇌ CH4 (g) + CCl4 (g) Kp = Kc Kp = 1.05 b) At 300 K, the reaction below has Kc = 279. What is Kp at 300 K? 2 SO2 (g) + O2 (g) ⇌ 2 SO3 (g) Δn = -1 therefore Kc 279 Kp 11.3 RT (0.082)(30 0) 3. Kp = 11.3 (3 points each) The reaction below has Kc = 12. What are the Kc values for the equilibria in a) and b)? Explain how you got your answer. CO(g) + H2O(g) ⇌ CO2(g) + H2(g) a) 2CO(g) + 2H2O(g) ⇌ 2CO2(g) + 2H2(g) Kc’ = Kc2 b) Kc = 144 CO2(g) + H2(g) ⇌ CO(g) + H2O(g) Kc’ = 1/Kc Kc = 0.083 2 4. a) (6 points) 2HI (g) ⇌ H2 (g) + I2 (g) A 2.00 L flask is filled with 0.300 mol of HI and allowed to reach equilibrium at a particular temperature. At equilibrium, [HI] = 0.104 M. Calculate Kc at this temperature. I C E 2 HI ⇌ H2 + I 2 0.150 M 0 0 -2x +x +x (0.150-2x) x x (0.150-2x) = 0.104 x = 0.023 [H2 ][I2 ] x2 (0.023)2 Kc [HI]2 (0.15 - 2x)2 (0.104)2 Kc 4.89 x 10 -2 b) (7 points) CO2 (g) + H2 (g) ⇌ CO (g) + H2O (g) (Kc = 2.4) 0.500 mol each of CO2 and H2 are placed in a 10.0 L flask and allowed to reach equilibrium at some temperature. Calculate the equilibrium concentrations of all species. CO2 + I 0.0500 C -x H2 ⇌ CO + H2O 0.0500 -x 0 0 +x +x E (0.0500-x) (0.0500-x) Kc 2.4 x2 (0.0500 - x) 2 x x x 0.0500 - x 1.549x 0.077 x 1.549 2.549x 0.077 5. x 0.030 [CO2 ] [H2 ] 0.020 [CO] [H2O] 0.030 (12 points) Consider the reaction in Question 4b again. CO2 (g) + H2 (g) ⇌ CO (g) + H2O (g) (Kc = 2.4) This time 0.500 mol of CO2 and 1.00 mol of H2 are placed in a 10.0 L flask and allowed to reach equilibrium at some temperature. Calculate the equilibrium concentrations of all species. CO2 + H2 ⇌ CO + H2O I 0.0500 0.100 0 0 C -x -x +x +x E (0.0500-x) (0.100-x) x x 2 x x2 K c 2.4 2.4 (0.0500 - x)(0.100 - x) (0.005 - 0.15x x 2 ) 2.4x2 – 0.36 x + 0.012 = x2 1.4 x2 = 0.36 x + 0.012 = 0 x x 0.36 0.1296 - 4(1.4)(0.012) 2.8 0.36 0.250 0.36 0.0624 2.8 0.039 or 0.218 2.8 We choose x = 0.039 [CO2] = 0.011M [H2] = 0.061M [CO] = [H2O] = 0.039M






