Periodic Table Game
advertisement

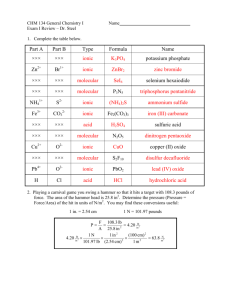
The Compound Game Review The Compound Game Is each compound ionic or molecular? Write the formula for each compound. Oxygen difluoride (molecular) OF2 (molecular) Iron(III) oxide (ionic) Fe2O3 sulfur trioxide SO3 (molecular) Potassium nitride K3 N (ionic) water H2 O Calcium acetate Ca(C2H3O2)2 (ionic) dinitrogen monoxide N2 O (molecular) Now try writing the name, given the formula (NH4 )2 SO4 Ammonium sulfate (ionic) AlCl3 (ionic) aluminum chloride SF6 (molecular) sulfur hexafluoride Pick up a reaction slip from the front. Work with one partner. Copy the reaction onto paper. • Label the type of reaction (synthesis, decomposition, single replacement, or double replacement) • Use coefficients to balance the equation. • Write the reaction as a word equation. • Fill in the parentheses with (s) or (aq) for any parentheses that are empty. • This is IDENTICAL to test part 2. 25.9 g/mol sodium nitride Na3N (ionic) 83.0 g/mol sulfur dibromide SBr2 (molecular) 191.9 g/mol dinitrogen pentoxide N2O5 (molecular) 108.0 g/mol copper(II) sulfide CuS (ionic) 95.7 g/mol calcium sulfide CaS (ionic) dioxygen difluoride O2F2 (molecular) P2O3 (molecular) diphosphorus trioxide dichlorine octoxide Cl2O8 (molecular) 199.0 g/mol