Calculating Empirical Formulas
advertisement

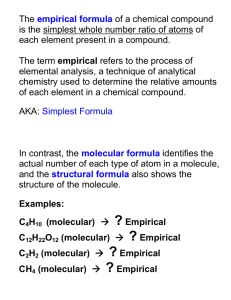
Calculating Empirical Formulas – Lowest whole-number ratio of the atoms of the elements in a compound • C6H12O6 (glucose) • The ratio that glucose normally has for carbon:hydrogen:oxygen is 6:12:6. • The lowest ratio that glucose has for carbon:hydrogen:oxygen is 1:2:1 (each number can be divided by the smallest number in the ratio which is 6). • The empirical formula for glucose is CH2O since this is the lowest wholenumber ratio of atoms for that compound. – May or may not be the same as the normal molecular formula of a compound • Next - Calculating empirical formulas What is the empirical formula of a compound that is 25.9% nitrogen and 74.1% oxygen? • If 25.9% of the compound is nitrogen and 74.1% of the compound is oxygen, then a compound with a mass of 100 g has 25.9 g of nitrogen and 74.1 g of oxygen. • To calculate the empirical formula, we need to relate the moles of each atom in the compound, so we need to convert the masses of the elements to moles. 25.9 g N 1 mol N mol N 1.85 mol N 1 14.0067 g N 74.1 g O 1 mol O mol O 4.63 mol O 1 15.9994 g O This would mean that the ratio of nitrogen to oxygen is N1.85O4.63. We can divide each number in the ratio of N1.85O4.63 by 1.85 to get N1O2.50. Since we cannot have 2.50 atoms of oxygen, we must multiply through each number by 2 to even it out, getting N2O5 as our empirical formula. • In calculating empirical formulas, remember that the number of atoms is a whole number. If the number of atoms for an element is close to a whole number (i.e., 2.1 or 2.2 or 2.8, or 2.9), you can usually round up or down to get a whole number. • If you should get a number of atoms closer to 2.33 or 2.5, multiply each number in the formula by a number that gets that to a whole number. For example, if you calculated 2.33, you would multiply this by 3 to get a value of 7 for that number. Empirical vs. Molecular Formula Calculating Molecular Formulas • Although sometimes a molecular formula may be the same as a molecule’s empirical formula, like in carbon dioxide (CO2), we have seen that the empirical formula for glucose is not the same as its molecular formula. • One can determine the molecular formula of a compound by knowing its empirical formula and its mass. • Next - Example Calculate the molecular formula of the compound whose molar mass is 180.1583 g and empirical formula is CH2O. We know that the molecular formula will have a molar mass of 180.1583 g. We also know, by calculating the gmm of CH2O, that CH2O has an empirical formula mass (efm) = 30.0264 g CH2O. Now, in order to figure out what we must multiply each number in the empirical formula by, we must figure out by what number we must multiply the empirical formula mass to get the molecular formula mass. To get from 30.0264 to 180.1583, 180.1583 30.0264 6 • Therefore, we must multiply each number of atoms in CH2O by 6 to get the molecular formula of C6H12O6. • You can double-check your answer by recalculating the molar mass of C6H12O6. • gmm C6H12O6 = 6 x 12.0111 g + 12 x 1.00794 g + 6 x 15.9994 g = 180.1583 g C6H12O6 • This agrees with the molar mass we were given, so the molecular formula we calculated is correct. Example (toughy) • 1.00 g of menthol on combustion yields 1.161 g of H2O and 2.818 g of CO2. What is the empirical formula? • Solution: – 1.161 g H2O 0.1298 g H = 0.1288 mol H – 2.818 g CO2 0.7690 g C = 0.0640 mol C – difference = 0.101 g O = 0.00632 mol O – ratios: H/O = 20.4 and C/O =10.1 – Therefore, C10H20O