1 - lkueh
advertisement

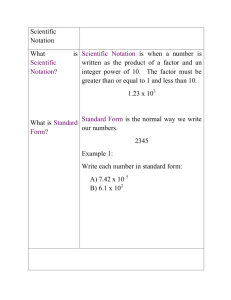
SCIENTIFIC NOTATION - LARGE NUMBERS 1. Complete the following table. The first line has been completed for you. Standard Form Product Form Scientific Notation 7 200 7.2 x 1000 7.2 x 103 45 000 8.5 x 10 000 1.1 x 100 000 9.78 x 108 2.03 x 107 2. State each value of n. a. 600 = 6 x 10n n= b. 7 100 000 = 7.1 x 10n c. 35 000 = 3.5 x 10n n= d. 54 000 000 = 5.4 x 10n n= f. 460 000 000 = 4.6 x 10n n= e. 145 000 = 1.45 x 10n n= n= 3. Write each number in scientific notation. a. 81 000 b. 300 000 c. 150 000 d. 200 000 000 e. 4 200 000 f. 71 300 000 g. 760 000 000 h. 5 020 000 000 4. Express each number in scientific notation: a. Coastline of Canada 91 000 km b. Distance across universe c. Speed of light 800 000 000 000 000 000 000 000 km d. Stars in our galaxy 120 000 000 000 e. Estimated age of Earth 4 500 000 000 years f. 1 300 000 0C Temperature of Sun’s interior 300 000 km/s 5. Why is 56 x 106 not in scientific notation? SCIENTIFIC NOTATION - SMALL NUMBERS 1. Write each number in scientific notation. a. 0.089 b. 0.2 c. 0.000 055 d. 0.0013 e. 0.000 000 101 f. 0.000 067 3. Express each number in scientific notation: a. Thickness of plastic 0.000 01 m b. Diameter of a hydrogen atom c. Diameter of a cell 0.000 000 005 cm d. Mass of a hydrogen atom 0.000 000 000 000 000 000 000 001 67 g d. Diameter of a diatom (one-celled plants that live in shells in the sea) 0.000 000 5 mm 0.000 001 m 4. Write in standard form. a. 1.8 x 105 b. 8.4 x 10-2 c. 2.24 x 10-2 d. 4.4 x 109 e. –3.02 x 10-3 f. 1.6 x 10-1 g. 1.88 x 10-5 h. –1.87 x 106 5. Do the following calculations. Write each answer in scientific notation. a. (3.4 x 105)(5 x 104) b. (2.5 x 10-3)(1.2 x 10-1) c. (5.2 x 103) (2 x 10-2) e. 870 600 000 x 710 000 5. One water molecule has a mass of about 3 x 10-26 kg. Calculate the number of water molecules in Lake Superior, which holds about 1.2 x 1016 kg of water.