Valence Electrons
advertisement

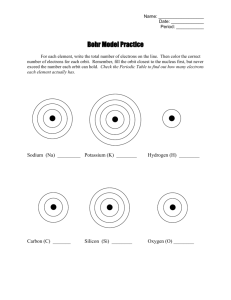
Valence Electrons As you go through the PowerPoint, answer the questions on your paper. • Make a section labeled REVIEW VIDEO. • As you watch the video, draw the example Bohr models. • Click the link below to watch the video. http://youtu.be/iih6rJ2S6pk Label the next section POWERPOINT QUESTIONS. Thinking about Bohr Models… 1.How many electrons can go on the first ring? 2.How many electrons can go on the second ring? 3.How many electrons can go on the third ring? 4. How many protons does lithium have? 5. How many neutrons? 6. How many electrons? 7. Draw the Bohr model for lithium. Use the picture below to check your answers to #7. If your Bohr model is wrong…go back and change it now! VALENCE = OUTSIDE Lithium has 3 total electrons but only 1 VALENCE ELECTRON. Valence electron Let’s practice… 8. How many valence electrons? • Did you get 6? 9. How many valence electrons? 10. How many valence electrons? 11. How many valence electrons? 12. Work out the Bohr model for magnesium and draw it on your paper. 13. How many valence electrons do you have on your model of magnesium? Label this section ELECTRON DOT DIAGRAMS. • Make sure you draw the example! • Click the link below to watch the video • http://youtu.be/y6QZRBIO0-o 14. Draw the dot diagram for hydrogen. 15. Draw the dot diagram for helium. 16. Draw the dot diagram for lithium. 17. Draw the dot diagram for sodium. 18. Draw the dot diagram for magnesium. 19. Draw the dot diagram for fluorine. 20. Draw the dot diagram for argon.