water only water and steam steam only
advertisement

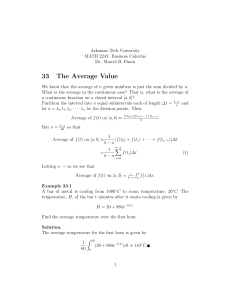
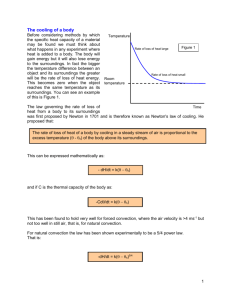
HEATING AND COOLING When a substance is being heated, you can tell that the particles are absorbing energy by measuring the temperature. For example, as you heat water, you should see a temperature increase as time goes by. But something odd will happen when the water starts to boil…. The temperature will stop increasing! The heating curve In this experiment, how long did it take for the water to start boiling? 4 minutes Why didn’t the temperature of the water increase after 4 minutes? Temperature (oC) water and steam 100 The energy was being used to separate the water molecules and turn the liquid into a gas. How long did it take for all the water to turn into steam? 12 minutes 18 4 16 Time (minutes) The cooling curve for stearic acid Temperature How can we find the freezing point of the acid? (oC) Look for where the temperature stops changing. This is where the substance is changing state. 60X 50 X X X X X 40 30 20 10 10 20 X X X The freezing point of stearic acid is 48oC. If this experiment was continued, the temperature would even off around 20oC. Why? When the solid stearic acid cooled to room temperature, it would not be able to cool any more. 30 Time (minutes) More cooling curves Objects lose heat because they are warmer than their surroundings. The hotter they are, in comparison to the environment, the faster they will lose heat energy (at the beginning anyway). Look carefully at the the two cooling graphs below and the following Which graph shows of 500g of answer water and which one questions. shows the cooling of 500g of solid iron? Explain your answer. temp temp A 100 100 75 75 50 50 25 25 time B B could not represent the cooling of water since the starting temperature is above 100oC time More cooling curves Objects lose heat because they are warmer than their surroundings. The hotter they are, in comparison to the environment, the faster they will lose heat energy (at the beginning anyway). Look carefully attemperature the two graphs and answer the following What was room on below the day this experiment was questions. done? How did you find your answer? temp temp A 100 100 75 75 50 50 25 25 time B Around 18oC. You can tell since this is where the graph shows that the objects temperature stopped changing. time More cooling curves Objects lose heat because they are warmer than their surroundings. The hotter they are, in comparison to the environment, the faster they will lose heat energy (at the beginning anyway). Look carefully the twocooled graphsfaster? below and the tell? following Which of theseatobjects Howanswer could you Why questions. do you think this one cooled faster? The iron would temp temp A B cool faster. You can tell since the 100 100 slope of graph B is steeper at the 75 75 start, than the 50 50 slope of graph A. Iron cools faster 25 25 since it is a better conductor. time time